Zika Virus – comprehensive review
Zika Virus
Clinical Microbiology Reviews 29:487-524, doi:10.1128/CMR.00072-15.
Didier Musso,a Duane J. Gubier b,c
Unit of Emerging Infectious Diseases, Institut Louis Malarde/Tahiti/ French Polynesia 3;
Program in Emerging Infectious Diseases, Duke-NUS Medical School, Singapore b;
Partnership for Dengue Control, Lyon, France c
📄 Download the 38 page PDF from Vitamin D Life
Some of the images
Many different species have carried Zika
How many species still carry Zika?
Range of Aedes and Albopictus
Text extracted from PDF
| SUMMARY | 488 |
| INTRODUCTION | 488 |
| ARBOVIRUSES: IMPORTANT CONCEPTS | 488 |
| History/Definition/Classification | 488 |
| Hosts/Reservoirs | 489 |
| Vectors and Transmission | 489 |
| Emergence | .489 |
| HISTORY AND EMERGENCE OF ZIKV | 489 |
| Discovery | .489 |
| ZIKV Serosurveys in the 1950s in Africa and Asia | 490 |
| Emergence of ZIKV in the Pacific | 490 |
| 2007: Yap State | 490 |
| 2013: French Polynesia | 490 |
| 2014: New Caledonia, Cook Islands, and Easter Island | 493 |
| 2015: Vanuatu, Solomon Islands, Samoa, and Fiji | .495 |
| Emergence of ZIKV in the Americas | 495 |
| Reemergence of ZIKV in Africa | 496 |
| Imported Cases of Zika Fever and Risk of Dissemination in Areas with Competent Aedes Mosquitoes | 496 |
| Europe | 496 |
| Americas | 497 |
| Asia | 497 |
| Pacific | 497 |
| The Disease Burden of ZIKV Infections | .497 |
| CLASSIFICATION AND PHYLOGENY OF ZIKV | ,498 |
| ECOLOGY | 499 |
| Host/Reservoir | 499 |
| Nonhuman primates | 499 |
| Other species | 499 |
| Vectors and Vector-Borne Transmission | 500 |
| Non-Vector-Borne Transmission | 502 |
| Laboratory contamination | 502 |
| Sexual transmission | 502 |
| Maternofetal transmission | 502 |
| Transfusion-transmitted infections | 502 |
| PATHOPHYSIOLOGY OF ZIKV INFECTIONS | 503 |
| Mechanisms of Infection | 503 |
| Replicative Cycle | 503 |
| Animal Studies | 503 |
| Cross Protection from ZIKV and Other Arboviruses | 503 |
| LABORATORY DIAGNOSIS OF ZIKA FEVER | 503 |
| Laboratory Safety | 503 |
| Clinical Laboratory Testing | 503 |
| Virus Detection | 503 |
| Antigen detection | 503 |
| Culture | 503 |
| Molecular detection | 504 |
| Molecular detection ofZIKV RNA | 504 |
| Detection ofZIKV in different body fluids | 504 |
| ZIKV RNA detection on filter paper | 504 |
| Serological Diagnosis | 505 |
| Diagnosis ofZika Fever in Countries Where It Is Endemic | 506 |
| Diagnosis ofTravelers | 506 |
| CLINICAL FEATURES OF ZIKA FEVER | 506 |
| Complications | 507 |
| Neurological complication in adults | 507 |
| Neurological complications in neonates | 509 |
| ZIKV-related death | 509 |
| Differential Diagnosis | 509 |
| Treatment | 509 |
| PREVENTION OF ZIKA FEVER | 510 |
| SURVEILLANCE OF ZIKA FEVER | 510 |
| THE POTENTIAL FOR ZIKV EMERGENCE | 510 |
| CONCLUSION | 512 |
| ACKNOWLEDGMENTS | 512 |
| REFERENCES | 512 |
| AUTHOR BIOS | 524 |
SUMMARY
Zika virus (ZIKV) is an arthropod-borne virus (arbovirus) in the genus Flavivirus and the family Flaviviridae. ZIKV was first isolated from a nonhuman primate in 1947 and from mosquitoes in 1948 in Africa, and ZIKV infections in humans were sporadic for half a century before emerging in the Pacific and the Americas. ZIKV is usually transmitted by the bite of infected mosquitoes. The clinical presentation of Zika fever is nonspecific and can be misdiagnosed as other infectious diseases, especially those due to arboviruses such as dengue and chikungunya. ZIKV infection was associated with only mild illness prior to the large French Polynesian outbreak in 2013 and 2014, when severe neurological complications were reported, and the emergence in Brazil of a dramatic increase in severe congenital malformations (microcephaly) suspected to be associated with ZIKV. Laboratory diagnosis of Zika fever relies on virus isolation or detection of ZIKV- specific RNA. Serological diagnosis is complicated by crossreactivity among members of the Flavivirus genus. The adaptation of ZIKV to an urban cycle involving humans and domestic mosquito vectors in tropical areas where dengue is endemic suggests that the incidence of ZIKV infections may be underestimated. There is a high potential for ZIKV emergence in urban centers in the tropics that are infested with competent mosquito vectors such as Aedes aegypti and Aedes albopictus.
INTRODUCTION
After the first isolation of Zika virus (ZIKV) in 1947 from a rhesus monkey (1), ZIKV infection in humans was first described in Nigeria (Africa) in 1954 (2). For half a century, fewer than 20 human infections were documented (3) and most of the data came from yellow fever virus (YFV) serosurveys. ZIKV was isolated from several mosquito species collected during arbovirus studies in Africa and during fever studies in Asia (1, 4-15). The first reported outbreak of Zika fever occurred in 2007 on the Western Pacific island of Yap in the Federated States of Micronesia (16); this was followed by a larger epidemic in French Polynesia in the South Pacific in 2013 and 2014 (17), with an estimated 30,000 symptomatic infections (18, 19). These epidemics were followed by smaller Pacific outbreaks in 2014 in New Caledonia (20), the Cook Islands (21), and Easter Island (22) and in 2015 in Vanuatu (23), the Solomon Islands (24), Samoa (25), and Fiji (26). In 2015, ZIKV emerged for the first time in the Americas (Brazil in March) and, as of the end of January 2016, autochthonous circulation of ZIKV has been reported in more than 20 countries or territories in South, Central, and North America and the Caribbean (24, 2732), and an outbreakwas reported in West Africa (Cape Verde) in November (33). The emergence of ZIKV was associated with the description of severe neurological complications: Guillain-Barre syndrome (GBS) in adults in French Polynesia and microcephaly in neonates in Brazil (31, 34-38).
Cocirculation of ZIKV with dengue virus (DENV) and chikungunya virus (CHIKV) has been documented in French Polynesia (39) and Brazil (27) but most likely also occurs throughout the Americas, Asia, several Pacific islands, and Africa, where DENV and CHIKV are endemic. It is now clear that ZIKV is following the path of DENV and CHIKV, spreading to all countries infested with Aedes aegypti and Aedes albopictus mosquitoes (40). Here we present a comprehensive review of the data available on this emerging virus.
ARBOVIRUSES: IMPORTANT CONCEPTS History/Definition/Classification
The term arbovirus, a contraction of arthropod-borne virus, is an ecological term defining viruses that are maintained in nature through biological transmission between a susceptible vertebrate host and a hematophagous arthropod such as a mosquito (41). Arboviruses were first classified according to serological criteria (antigenic classification) (41-44). A new molecular basis for taxonomy is now used, and the genus Flavivirus is classified in clusters, species, and clades (45-48). The genus Flavivirus is composed of 53 virus species placed in three clusters: mosquito-borne viruses, tick-borne viruses, and viruses with no known vector (International Committee on Taxonomy of Viruses website chapter on virus families not assigned to an order, family Flaviviridae, http://ictvonline.org/virusTaxonomy.asp) (45, 49, 50). A fourth group of viruses found only in insects will also likely be placed in this genus (51). For additional information on the history, definition, classification, taxonomy, and diagnosis of arboviruses, see previously published reviews (51-56).
Hosts/Reservoirs
Most of the arboviruses cause zoonoses that usually depend on nonhuman animal species for maintenance in nature. Many animal species are host reservoirs (host of an infection in which the infectious agent multiplies and develops and on which the agent is dependent for survival in nature) of arboviruses (57,58); humans, with few exceptions (DENV, CHIKV, or YFV) are dead-end or accidental hosts (hosts from which infectious agents are not transmitted to other susceptible hosts) (59). Arboviruses such as DENV have adapted completely to humans and can be maintained in large tropical urban centers in a mosquito-human-mosquito transmission cycle that does not depend on nonhuman reservoirs (57). However, sylvatic strains of DENV still occur and can infect humans, suggesting the possibility of reemergence of DENV from sylvatic cycles; arboreal mosquitoes are also capable of transmitting human DENV strains (60-62).
Vectors and Transmission
A vector of arboviruses maybe defined as an arthropod that transmits the virus from one vertebrate to another by bite (63). The most common mode of biological transmission is infection during a viremic blood meal and injection of infectious saliva during blood feeding (horizontal transmission).
Nonvector arbovirus transmission has been reported to occur directly between vertebrates (64, 65), from mother to child (6671), nosocomially (72-74), by transfusion (75-78), via bone marrow (79) or organ (80) transplantation, and sexually (81).
Emergence
In the last 40 years, there has been a resurgence of a number of well-known arboviruses (57), such as West Nile virus (WNV), DENV, and CHIKV. The capacity of arboviruses to adapt to new vectors may have a major impact on the geographic expansion of arboviruses. For example, DENV, YFV, and CHIKV can be transmitted by feral African, Asian, or American mosquitoes but have adapted to domesticated Ae. aegypti and Ae. albopictus (82). Other factors associated with the emergence of arboviruses include (57, 83) genetic changes for CHIKV (84-87), DENV (88-91), and WNV (92-94); climate change (95-97); uncontrolled use of insecticides (98); perturbations of natural systems that are frequently anthropogenic (97, 99, 100); expansion of the geographic distribution of mosquito vectors (101, 102); adaptation to new reservoir/amplification hosts (103); global growth of human populations with extensive urbanization (57, 95); lack of effective mosquito control (104); and increased travel (57, 105). We have presented only a few examples of arbovirus emergence, for additional data, see reviews of arbovirus emergence, especially those by Gubler (57), Kuno and Chang (65), Powers (83), Weaver et al. (95, 106), and Vazilakis et al. (107).
HISTORY AND EMERGENCE OF ZIKV
The discovery of ZIKV and many other arboviruses was the result of research programs on yellow fever sponsored by the Rockefeller Foundation from 1914 to 1970. ZIKV was discovered in the course of a study of the vector responsible for the cycle of sylvan YFV in Uganda (1,108-110). Over a 10-year period from 1937 to 1947,10 different viruses were isolated at the Yellow Fever Research Institute, Entebbe, Uganda, including 7 new viruses (108): WNV (111) and Bwamba virus (112) in 1937, Semliki Forest virus in 1942 (113), Bunyamwera virus (114) and Ntaya virus (115) in 1943, and Uganda S virus (116) and ZIKV (1, 117) in 1947. With the exception of the Uganda S virus, all of these viruses were named after the geographic places where they were isolated. Four of these viruses were related, belonging to the genus Flavivirus (WNV, Ntaya virus, Uganda S virus, and ZIKV) (45). There are considerable data on the seroprevalence of ZIKV in Africa, but because of the large number of flaviviruses in that region and the extensive cross-reactivity among the viruses of that genus, the data are difficult to interpret. The fact that these viruses were discovered in Uganda does not necessarily reflect the origin of the viruses but rather indicates areas in Uganda where yellow fever studies were conducted.
Discovery
In April 1947, six sentinel platforms containing caged rhesus monkeys were placed in the canopy of the Zika Forest of Uganda (1). On 18 April, the temperature of one of the caged rhesus monkeys (no. 766) was 39.7°C. A blood sample was taken from that monkey on the third day of fever and injected intracerebrally and intraperitoneally into Swiss mice and subcutaneously into another rhesus monkey (no. 771). All of the mice inoculated intracerebrally showed signs of sickness on day 10 after inoculation, and a filterable transmissible agent was isolated from the brains of those sick mice. During the observation period, monkey no. 766 showed no abnormality other than pyrexia and monkey no. 771 showed neither an elevated body temperature nor any other abnormality. The agent isolated from monkey no. 766 was referred to as ZIKV (the ZIKV 766 strain). This agent was neutralized by convalescent-phase serum taken from monkey no. 766 1 month after the febrile episode and by serum taken from monkey no. 771 35 days after inoculation. Preinfection serum samples collected from these monkeys did not neutralize the ZIKV 766 strain.
In January 1948, mosquitoes were collected in the Zika Forest in an attempt to isolate YFV (1). Eighty-six Aedes africanus mosquitoes were collected, and mice were inoculated with the Seitz filtrate of pools of these mosquitoes. One mouse died on day 6 after inoculation, and one appeared sick on day 14. The virus isolated from Ae. africanus was designated ZIKV (E/1 strain). The remaining portion of the Seitz filtrate was inoculated subcutaneously into rhesus monkey no. 758. This monkey remained asymptomatic, but two mice inoculated intracerebrally with blood taken from this monkey died and another became sick; ZIKV (758 strain) was isolated from its serum. Rhesus monkey no. 758 developed neutralizing antibodies to the agent isolated from its serum, to the strain ofvirus isolated from Ae. africanus (ZIKV E/1 strain), and to the strain isolated from rhesus monkey no. 766 (the ZIKV 766 strain). Cross neutralization tests (NT) showed that ZIKV was different from YFV, DENV, and Theiler’s encephalomyelitis virus; NT with ZIKV and the antisera from other neurotropic viruses showed no relationship. Cross-reactions performed by complement fixation (CF) confirmed that ZIKV was a distinct virus (118).
The first human ZIKV isolate came from a 10-year-old Nigerian female in 1954 (2). ZIKV was isolated in mice inoculated with the patient’s serum. Interpretation of the clinical presentation of the patient was difficult because the patient’s blood also contained numerous malaria parasites. The other two cases of human ZIKV infection reported in 1954 in Nigeria were confirmed by a rise in serum neutralizing antibodies (2). Outside Africa, ZIKV was isolated for the first time from mosquitoes (Ae. aegypti) in 1969 in Malaysia (4); subsequently, the first human infections were reported in central Java, Indonesia, in 1977 (119).
ZIKV Serosurveys in the 1950s in Africa and Asia
Serosurveys for arboviruses were conducted by using a hemagglutination inhibition (HI) test (120), a CF test (121), an NT (117), a mouse protection test (2), a hemagglutination assay (122), and an enzyme-linked immunosorbent assay (ELISA) (123). The HI test described by Clarke and Casals (124) has been the most extensively employed (52).
Interpretation of Flavivirus serological results is difficult because cross-reactions within this group of arboviruses were not well characterized when the first serosurveys were conducted. Discrepant results were observed when sera were tested by different methods (125-127) and even when the same method was used (128). Some studies reported results only for “arbovirus group B,” but results for ZIKV were not available (129). ZIKV was not always included in the panel of antigens tested. For example, several studies of both human and animal sera in South Africa included only serological data for Spondweni virus (SPOV), the Flavivirus closest to ZIKV; positive samples were found (130,131). Because of cross-reactions within the Flavivirus genus, positive reactions for SPOV could have been the consequence of cross-reactions with ZIKV or another Flavivirus.
Nevertheless, although the data should be interpreted with caution, serosurveys suggest that ZIKV is endemic to Africa (East, Central, West, and South) and several countries in Asia. These global data were further confirmed by isolation of ZIKV from vectors and vertebrate hosts in most of these countries. Detailed results of ZIKV serosurveys of humans are reported in Table 1. African, Asian, American, and Pacific countries in which ZIKV strains or ZIKV antibodies have been detected in humans, animals, or vectors are shown in Fig. 1 to 4, respectively.
Emergence of ZIKV in the Pacific
2007: Yap State. Yap State is one of four states in the Federated States of Micronesia, located in the Western Pacific. The population of Yap State is about 7,500 (2000 census data). In April and May 2007, local physicians reported an outbreak of “dengue-like illness.” An outbreak of dengue fever was suspected, as this virus had previously occurred in Yap State in 1995 (132) and 2004 (133). Three patients tested positive for DENV with rapid commercial DENV immunoglobulin M (IgM) kits (134), but the physicians had the impression that the illness was different from dengue fever because, in addition to rash and arthralgia, which are common in dengue, some patients also reported only subjective fever and conjunctivitis (Secretariat of the Pacific Community, http://www.spc.int/phs/english/publications/informaction/IA27 /Zika-outbreak-Yap-2.pdf). Acute-phase serum samples collected from 71 patients were sent to the Centers for Disease Control and Prevention (CDC) Arbovirus Diagnosis and Reference Laboratory in Fort Collins, CO, USA. ZIKV RNA was detected in 10 samples (14.1%). Laboratory investigations included ELISA for IgM antibodies to ZIKV, determination of neutralizing antibody titers, and RNA detection by a specific ZIKV reverse transcription (RT)-PCR assay of acute-phase samples (16, 135).
One hundred eighty-five cases of suspected Zika fever (symptoms of Zika fever without laboratory confirmation) were investigated; 49 (26.5%) were confirmed (suspected cases with laboratory confirmation), 59 (31.9%) were probable (suspected cases with equivocal laboratory results), and 72 (38.9%) remained suspected Zika fever. ZIKV RNA was detected in 15 (33.3%) of the 45 serum samples collected from patients before day 10 after the onset of illness. A serosurvey of 173 selected households was conducted; 414/557 (74.3%) persons had IgM antibodies to ZIKV, and 156 (37.7%) of them were symptomatic. However, 18.9% of the patients with no detectable IgM antibodies to ZIKV also reported symptoms compatible with Zika fever. ZIKV was not isolated from any of the patients.
An estimated 5,005 (72.6%) of the 6,892 residents over 3 years old were infected with ZIKV, and an estimated 919 or 18.4% (95% confidence interval [CI],480to 1,357) of the infected patients had a clinical illness that was probably attributable to ZIKV infection. The relative risk of males versus females was 1.1 (95% CI, 1.0 to 1.2). The clinical attack rate of Zika fever was higher among females and older persons, but the prevalence of specific IgM antibodies was higher in males (relative risk, 1.1) and did not vary significantly across age groups. No behavioral or environmental risks factors were associated with ZIKV infection. The duration of the outbreak was about 3 months. The origin of the ZIKV that caused the Yap State epidemic remains unknown, but introduction by a viremic person from the Philippines was suspected because of evidence of ZIKV infections in humans in that country and frequent travel exchange between Yap State and the Philippines. It is speculated that anew strain of ZIKV with greater fitness and epidemic potential emerged to cause this epidemic in the same manner that epidemic strains of DENV have emerged in recent decades (88, 91). This was the first detection of ZIKV outside Asia and Africa and the first large ZIKV outbreak ever reported. Before this outbreak, only 14 human infections had been reported (3). This outbreak underscored the potential of ZIKV as a newly emerging arbovirus.
2013: French Polynesia . French Polynesia is a French overseas territory in the South Pacific. The population is about 270,000 (2012 census) living on 67 islands distributed among five archipelagoes. French Polynesia is tropical, with a dry season (May to October) and a rainy season (November to April). Until 2013, DENV was the only arbovirus detected in French Polynesia, causing multiple outbreaks since the 1960s (39,136-138). However, a retrospective serosurvey of serum samples collected from 2011 to 2013 supported the existence of silent autochthonous circulation of Ross River virus (RRV) (139).
In October 2013, patients from the same family presented with a “dengue-like illness” with low fever (<38°C), asthenia, wrist and finger arthralgia, headache, and rash; two of them had conjunctivitis, and one had swollen ankles and aphthous ulcers. The patients tested negative for DENV, CHIKV, and WNV by specific RT-PCR. Because of past circulation of ZIKV in the Pacific, they were also tested for ZIKV, but the RT-PCR results were equivocal. Two weeks later (week 43), another patient reporting similar symptoms tested positive by a specific ZIKV RT-PCR (135); results were confirmed by RNA sequencing of the prM/E protein codingregion (17). Concomitantly, the French Polynesia Ministry of Health reported an increase in patients visiting primary care physicians with dengue-like syndrome and rash. By week 51, an estimated 19,000 suspected cases were recorded; 294/584 were positive by a specific ZIKV RT-PCR test (17).
TABLE 1 Human ZIKV serosurvey
assay. c Study of DENV-2-immune serum samples.
d Details are not available for ZIKV, but ZIKV antibodies were detected in the 21 areas where samples were collected. e Positive sera in 18/38 localities.
f Reactive only for ZIKV; number broadly reactive not reported. g Monoreactive for ZIKV. h Group B arboviruses, including ZIKV.
1 Two of these seroconverted. j ZIKV antibodies. k ZIKV and DENV-2 antibodies. l ZIKV and two or more other antibodies. m Mosquito catchers. n Suspected cases of yellow fever.
0 Monoreactive for ZIKV. p Blood donors.
q All serum samples collected after ZIKV outbreak. r Thirty-eight scattered localities.
The Institute Louis Malarde (Health and Research Institute of Tahiti, French Polynesia) tested 855 patients presenting symptoms of Zika fever (1,067 samples) for ZIKV RNA; 392 were positive (140). The duration of the outbreak was about 21 weeks, peaking on week 9 of 2014 with an estimate of 3,500 consultations for Zika fever (Direction de la Sante de la Polynesie Franaise, http://www.hygiene-publique.gov .pf/) (Fig. 5). All of the archipelagoes of French Polynesia were affected. At the end of the outbreak, the estimated number of cases of Zika fever was 30,000 (11.5% of the population) (18, 19, 141). The magnitude of the outbreak was likely due to the low level of preexisting immunity to ZIKV in the population and the high densities of competent mosquito vectors (123). However, the total number of infections remains unknown because most patients with mild Zika fever did not seek medical care and there were probably many asymptomatic patients.
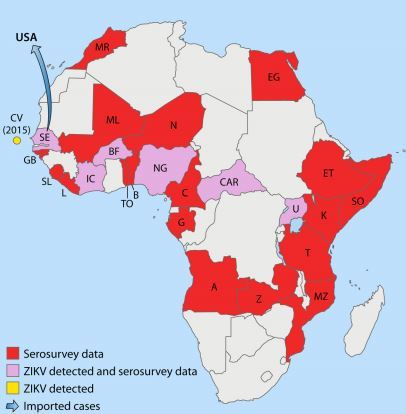
FIG 1 African countries in which ZIKV circulation has been reported up to January 2016
. Abbreviations: MR, Morocco; CV, Cape Verde; SE, Senegal; GB, Gabon; SL, SierraLeone; L, Liberia; IC, Ivory Coast; BF, BurkinaFaso; ML, Mali; N, Nigeria; NG, Niger; TO, Togo; B, Benin; C, Cameroon; CAR, CentralAfrican Republic; G, Gabon; A, Angola; Z, Zaire; MZ, Mozambique; T, Tanzania; U, Uganda; K, Kenya; SO, Somalia; ET, Ethiopia; EG, Egypt.
Severe neurological complications and non-vector-borne transmission were described in a small percentage of the cases. A serosurvey of all age groups conducted after the outbreak suggested an infection rate of 50 to 66% (142). The origin of the ZIKV in French Polynesia is unknown, although it was closely related to the strains isolated in Yap State in 2007 and in Cambodia in 2010 (17). During and after the French Polynesia outbreak, ZIKV spread rapidly to other Pacific islands (18) (Fig. 4).
2014: New Caledonia, Cook Islands, and Easter Island . New Caledonia, another French overseas territory in the South Pacific, only had DENV and CHIKV arbovirus transmission prior to the introduction of ZIKV. Three cases of Zika fever were reported in New Caledonia in patients returning from French Polynesia at the end ofNovember 2013 (143,144). By the middle of January 2014, 26 imported cases from French Polynesia had been confirmed and the first autochthonous transmission was documented (20, 144). A ZIKV outbreak was declared in February, and by the end of August, about 1,400 confirmed cases had been reported, including 35 imported cases (32 from French Polynesia, 2 from Vanuatu, and 1 from the Cook Islands) (141, 144, 145). ZIKV was still circulating in this country in 2015, with 137 confirmed cases reported through August (Direction des Affaires Sanitaire et Sociales de Nouvelle-Caledonie, http://www.dass.gouv.nc/portal/page /portal/dass/) (24). The duration of the outbreak was 29 weeks, with a peak at week 14. The clinical presentation of ZIKV in New Caledonia was similar to that observed in French Polynesia. Interestingly, two coinfections (DENV and ZIKV) were reported during the New Caledonia outbreak, and both patients recovered after a mild clinical course (144). A comparison of the French Polynesian (Direction de la Sante de la Polynesie Franaise, http: //www.hygiene-publique.gov.pf/) and New Caledonian (Direction des Affaires Sanitaire et Sociales de Nouvelle-Caledonie, http: //www.dass.gouv.nc/portal/page/portal/dass/) epidemic profiles is shown in Fig. 5. The number of confirmed cases of ZIKV infection in New Caledonia was 1,400 or about 0.8% of the population, compared to 11.5% of the population of French Polynesia. Several mechanisms can explain the difference in the epidemiological profiles: different populations (mainly Polynesian, European, and Chinese in French Polynesia and Melanesian and European in New Caledonia), different mosquitoes (Ae. aegypti and Aedes polynesiensis in French Polynesia and Ae. aegypti in New Caledonia), and different climates (lack of a cold season in French Polynesia, cold season in New Caledonia), different vector control strategies, and a change in the virus epidemic potential. The same difference was observed in the chikungunya outbreaks that occurred in New Caledonia in 2011 (33 autochthonous cases), 2013 (30 autochthonous cases), and 2014 (2 autochthonous and 27 imported cases) (25) and in French Polynesia in 2014 and 2015 (66,000 cases or about 25% of the population) (25). The outbreak profiles were related to neither population size (about 270,000 inhabitants for both countries) nor background immunity (both were naive to ZIKV). However, the possibility of microgenetic changes causing phenotypic changes, such as those that occur with dengue (88, 91), cannot be excluded, and microevolution of ZIKV during outbreaks has been reported (146).
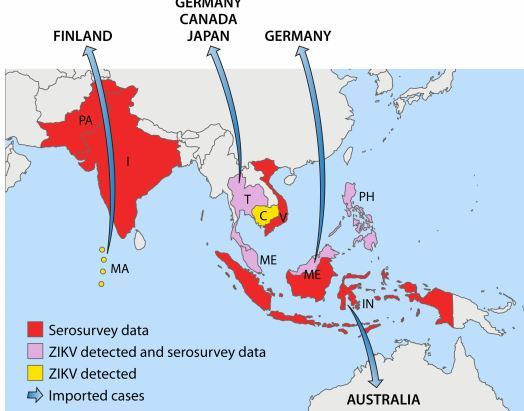
FIG 2 Asian countries in which ZIKV circulation has been reported up to January 2016
Abbreviations: PA, Pakistan; I, India; T, Thailand; C, Cambodia; V, Vietnam; MA, Maldives; ME, Malaysia; PH, Philippines; IN, Indonesia.
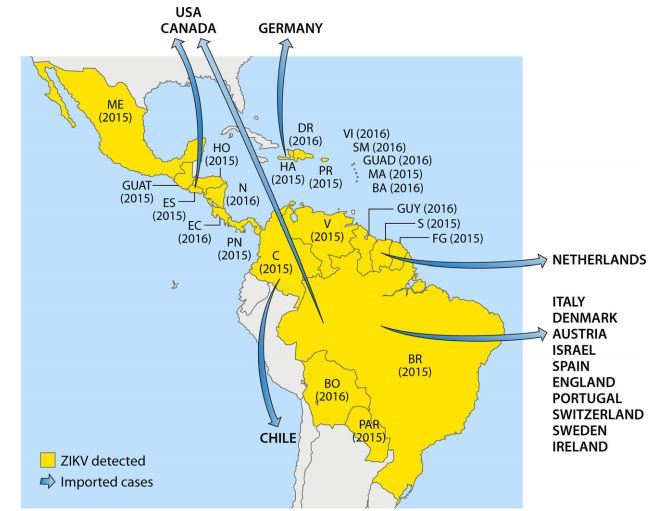
FIG 3 American countries in which ZIKV circulation has been reported up to January 2016.
Abbreviations: ME, Mexico; DR, Domincan Republic; VI, Virgin Islands; SM, Saint Martin; GUAD, Guadeloupe; MA, Martinique; BA, Barbados; HA, Haiti; PR, Puerto Rico; HO, Honduras; GUAT, Guatemala; N, Nicaragua; ES, El Salvador; EC, Costa Rica; PN, Panama; V, Venezuela; GUY, Guyana; S, Suriname; FG, French Guiana; C, Colombia; BR, Brazil; BO, Bolivia; PAR, Paraguay.
A ZIKV outbreak was declared in March 2014 in the Cook Islands after laboratory confirmation of 18 cases by the Institute Louis Malarde, French Polynesia (Institut National de Veille Sani- taire Franqais, http://www.invs.sante.fr/Publications-et-outils /Bulletin-hebdomadaire-international). It was a small outbreak, with only 905 cases reported, 49 of which were confirmed as ZIKV infections (21, 147). The first case was that of a traveler returning from French Polynesia (147). In the Cook Islands, DENV is endemic (148).
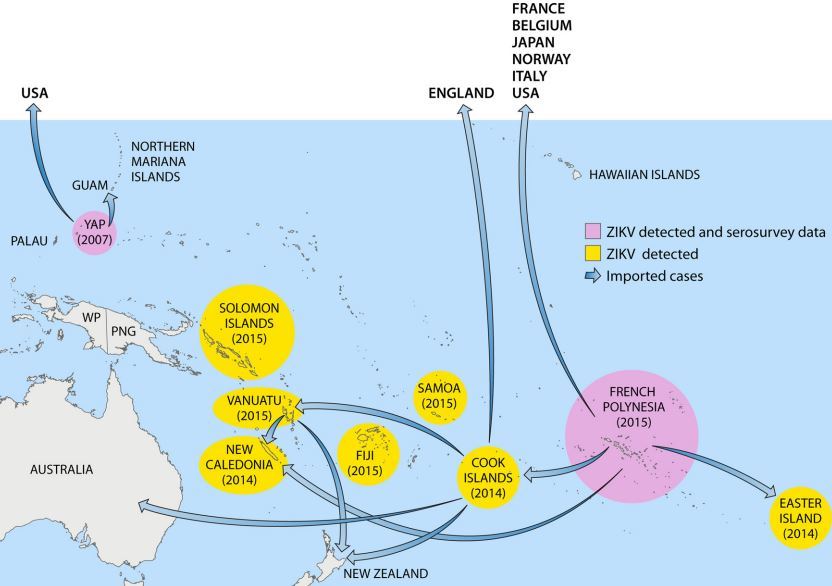
FIG 4 Pacific countries in which ZIKV circulation has been reported up to January 2016
. Abbreviations: WP, West Papua; PNG, Papua New Guinea.
The Chilean Ministry of Health confirmed the first autochthonous case of ZIKV infection on Easter Island on 28 January 2014 (National Travel Health Network and Centre, http://nathnac .net/). By early March, 40 suspected cases were reported (Institut National de Veille Sanitaire Franais, http://www.invs.sante.fr /Publications-et-outils/Bulletin-hebdomadaire-international) (22, 147). Fifty cases were ultimately confirmed by the Public Health Institute of Chile (149). It was suggested that ZIKV was introduced to Easter Island during the annual Tapati Festival, which attracted people from other Pacific islands, especially French Polynesia, where a ZIKV outbreak was ongoing (22,150). Dengue epidemics occurred on Easter Island in 2000 and 2002 (151,152).
FIG 5 French Polynesian and New Caledonian outbreak profiles.
2015: Vanuatu, Solomon Islands, Samoa, and Fiji. Very few data are available from Vanuatu (23, 24). An unspecified number of confirmed cases of ZIKV infection were reported by health officials and by the European Centre for Disease Prevention and Control (ECDC) in the weeks after tropical cyclone Pam passed through Vanuatu in March 2015 (24) (Auckland Regional Public Health Services, http://www.arphs.govt.nz/health-information /communicable-disease/dengue-fever-zika-chikungunya# .VeqEFv8Vipo). Circulation of ZIKV in Vanuatu was supported by the export of three cases from there, one to New Zealand (153) and two to New Caledonia (144). Zika fever was confirmed in the Solomon Islands in March, with 310 cases reported to date (Auckland Regional Public Health Services, http://www.arphs.govt.nz/health-information/communicable -disease/dengue-fever-zika-chikungunya#.VeqEFv8Vipo; Solomon Islands Broadcasting Corporation, http://www.sibconline.com.sb /health-authorities-declare-outbreak-of-new-virus-in-solomons/)(24). In Samoa (25) and Fiji (26), ZIKV circulation has been reported but the number of cases is not available. DENV is endemic to all of these countries (154).
Because the clinical symptoms of Zika fever overlap those of DENV and CHIKV infections, it is likely that ongoing and undetected ZIKV transmission in the Pacific islands will continue (18, 155).
Emergence of ZIKV in the Americas
ZIKV is a new threat to the Americas (156-168).
Clusters of “exanthematic diseases” have been reported retro-spectively in Brazil since late 2014 (169), and since February 2015, an outbreak of “exanthematic disease” has affected thousands of patients in northeastern Brazil, mainly in BaMa, Maranhao, Pernambuco, Rio Grande do Norte, Sergipe, and Parafoa (24, 27, 28, 170). In March, serum samples obtained from patients with a presumptive diagnosis of acute viral illness at Santa Helena Hospital in Camarap, BaMa, Brazil, were analyzed at the Federal University of BaMa (27) and at the Molecular Virology Laboratory of the Carlos Chagas Institute, Oswaldo Cruz Institute, State of Panara, Brazil (28). Alerts were issued by the Brazilian Ministry of Health and the Pan American Health Organization (PAHO) (171), and on 15 May, the first autochthonous Zika fever was confirmed in a patient from BaMa (172). As of early December 2015, 18 states in Brazil have confirmed autochthonous virus transmission in the northern, northeastern, southeastern, central western, and southern regions (37): Alagoas (173-175), BaMa (27, 172, 176), Ceara (177), Maranhao (24), Mato Grosso (175, 178), Para (173), Parafoa (179), Parana (180, 181), Pernambuco (179), Piam (182), Rio Grande do Norte (28), Rio de Janeiro (183), Roraima (184), Sao Paulo (185),Esphito Santo (37), Amazonas (37), Rondonia (37), and Tocantins (37). However, ZIKV was probably already circulating in other states but had not yet been detected. In late December 2015, the estimated number of suspected cases of ZIKV infection ranged from 440,000 to 1,300,000 (32).
Many arboviruses are endemic to Brazil. The four DENV serotypes circulate and affect all Brazilian regions (186, 187). Over 1 million cases of dengue were reported annually between 2009 and 2012, and 2.3 million cases were reported in 2013 (PAHO, http: //www.paho.org/hq/index.php?option=com_topics). YFV, Saint Louis encephalitis virus, Mayaro virus, and Oropouche virus also circulate in some parts of Brazil (188). CHIKV strains belonging to the Asian (189) and East, Central, and South African (190) lineages have circulated in Brazil since September 2014.
The potential for ZIKV emergence in Brazil was great because Ae. aegypti and Ae. albopictus have a widespread distribution (164): Ae. aegypti is dispersed in Brazil, especially in the northern, northeastern, and central eastern regions (191, 192), but populations of Ae. albopictus are larger in subtropical areas, especially in southern Brazil (193). It was first suggested that ZIKV was introduced to Brazil during the World Cup soccer competition in 2014 (28, 172, 194), but teams from Pacific countries with ongoing circulation of ZIKV did not participate in that competition. In 2014, however, Brazil also hosted the World Spring Canoe championship in Rio de Janeiro with the participation of four Pacific Countries in which ZIKV was circulating (French Polynesia, New Caledonia, the Cook Islands, and Easter Island). Phylogenetic studies suggest that ZIKV may have been introduced to Brazil during that sporting event (150). Brazil will host the summer Olympic and Paralympic games in Rio de Janeiro in August 2016. With about 500,000 people expected to visit Brazil for that competition, there will be an increased risk of ZIKV spread around the world by those travelers.
In October 2015, ZIKV infections were confirmed in Colombia in the state of Bolivar (179,195, 196) and subsequently spread to other states (30,197-204), with an estimate of about 14,000 cases as of early January 2016 (31). In late 2015, autochthonous ZIKV circulation was reported in 12 other countries and territories of the Americas and the Caribbean: Suriname (29, 33), Venezuela (26), Guatemala (205), Honduras (206), Mexico (205), Paraguay (207), Panama (206, 208) French Guiana (206), El Salvador (37,209), Haiti (210), Puerto Rico (210), and the French Caribbean (Martinique) (206). By January 2016, ZIKV was also detected in Bolivia (211), Nicaragua (209), Guyana, and Ecuador (209) in South America and in Barbados, the Dominican Republic, Guadeloupe, and the U.S. Virgins Islands in the Caribbean (209). ZIKV is probably already circulating in other American countries but has not been detected because of misdiagnosis as other arboviruses and a lack of laboratory facilities. This report covers the geographic spread of ZIKV only through late January 2016, but the spread is still ongoing.
Reemergence of ZIKV in Africa
Until 2015, only sporadic ZIKV infections were reported in Africa. In November 2015; however, the epidemic strain of ZIKV returned to the geographic origin of its discovery (Africa) when the Ministry of Health of Cape Verde reported a ZIKV outbreak. Seventeen of the 64 serum samples sent to the Pasteur Institute of Dakar, Senegal, tested positive for ZIKV and negative for CHIKV and DENV (33). About 5,000 suspected cases were reported from September to December 2015 (WHO, http://www.who.int/csr /don/21-december-2015-zika-cape-verde/en/#). Cape Verde is close to Senegal, where ZIKV is endemic (7), but it is likely that the Cape Verde outbreak is related to the tourist industry on that island, especially because many Brazilians regularly travel to Cape Verde for vacations. Of note is that the island is infested with Ae. aegypti and that a DENV outbreak occurred there in 2009, with more than 17,000 cases reported (212).
Imported Cases of Zika Fever and Risk of Dissemination in Areas with Competent Aedes Mosquitoes
Imported cases of Zika fever have been reported in travelers returning from areas with endemic/epidemic Zika fever (Fig. 1 to 4). These importations increase the risk of dissemination of ZIKV to areas where potential competent vectors are present, especially Ae. aegypti and Ae. albopictus.
Europe . The first imported case of Zika fever in Europe was reported in a German traveler infected in Thailand in November 2013 (213). Other German cases were reportedly imported from Malaysian Borneo in September 2014 (214) and from Haiti in December 2015 (210). Ae. albopictus has a limited distribution in Germany (215), and Ae. aegypti is not present (ECDC, http://www .ecdc.europa.eu/en/healthtopics/vectors/).
Three ZIKV infections imported from French Polynesia have been reported in France, the first in November 2013 (216); data are not available for the other two cases (217). Ae. albopictus was first detected in France in 2004 (101); it is now well established in the southern part of the country, where it was responsible for local transmission of dengue (102, 218) and chikungunya (219). Ae. aegypti is not present in continental France (ECDC, http://www .ecdc.europa.eu/en/healthtopics/vectors/). As ZIKV is now endemic to the French Caribbean islands of Martinique, Guadeloupe, and Saint Martin and to French Guiana in South America, introductions are occurring (220) in continental France, with a risk of autochthonous transmission if introductions occur during the hot season (when Ae. albopictus circulates in France). However, large outbreaks are not expected.
Three imported ZIKV infections have been reported in Italy, two from French Polynesia in January 2014 (221) and one from Brazil (BaMa) in March 2015 (222). The potential for arbovirus outbreaks in Italy was demonstrated by the CHIKV outbreak in the province of Ravenna (region of Emilia Romagna) in 2007 (223-225). That region has also experienced WNV outbreaks (226, 227). Ae. albopictus was first detected in Italy in 1990 (228), and Italy is now the European country most heavily infested with this species (229).
ZIKV infection was reported in a Norwegian traveler infected during a 14-day trip to French Polynesia in December 2013 (230) and in Netherlands travelers returning from Suriname in 2015 (31), but both countries are free of Ae. aegypti and Ae. albopictus (230).
Other imported cases of ZIKV have been reported in Denmark, Finland, Austria, Switzerland, Israel, Spain, Ireland, Sweden, England, and Portugal (209, 220). The highest risk of local transmission is in Spain, which is infested with Ae. albopictus (231).
European overseas tropical and subtropical countries and territories infested with Ae. albopictus, Ae. aegypti, and/or other species of Aedes mosquitoes in the Mediterranean, the Atlantic Ocean, the Caribbean, South America, and the Indian Ocean are at high risk of ZIKV infection because DENV and/or CHIKV are already endemic to most of these areas (24).
Americas . In 2007, a medical volunteer visited Yap State during the outbreak and developed symptoms of ZIKV infection and antibodies to ZIKV were detected after that person returned to the United States (16). Two American scientists developed Zika fever in Colorado a few days after being infected while performing a mosquito-sampling project in southeastern Senegal in August 2008 (232), a case imported from French Polynesia has been reported in Texas (233), and another has been reported in New York City (234); other imported cases have been reported in Arkansas, Florida, Hawaii, Illinois, New York, Texas, and Virginia (209, 235). The risk of secondary transmission is highest in states such as Texas and Florida, where both Ae. albopictus and Ae. aegypti are present (236), and autochthonous cases of dengue fever and/or chikungunya have occurred (CDC, http://www.cdc.gov/chikungunya/geo/united-states-2014 .html) (237-239).
To our knowledge, Hawaii reported neither imported nor autochthonous ZIKV infections during the French Polynesian outbreak, despite probable ZIKV introduction because of weekly flights between Tahiti (French Polynesia) and Hawaii, where Ae. albopictus is present and caused a small outbreak of DENV in 2001 (240).
ZIKV infection was reported in a Canadian traveler returning from Thailand in February 2013 (241, 242). Ae. albopictus has been rarely detected on the West Coast of Canada (215, 243).
Chile reported no cases of ZIKV imported from Easter Island during the outbreak there in 2015, but one case was imported from Colombia in December 2015 (211).
Asia . Zika fever was reported in a Japanese traveler returning from Thailand in August 2014 (244, 245), although the diagnosis was based on serology only, suggesting a possible cross-reaction with DENV (246). Two other cases, both confirmed by RT-PCR, were reported after travel to French Polynesia in December 2013 and January 2014 (247). Japan experienced dengue outbreaks during World War II, but any introduction now will likely remain focal (248), as was the recent outbreak of dengue in Tokyo that was caused by Ae. albopictus (249). ZIKV is endemic to many countries in Asia, and they are at risk of ZIKV outbreaks.
Pacific . Forty-five ZIKV infections were reported in 2014 in New Zealand (250, 251), 43 of which involved a history of travel to the Cook Islands, where a ZIKV outbreak was ongoing; one case was imported from Vanuatu (153). Autochthonous arbovirus infections have never been reported in humans in New Zealand (153). Ae. albopictus and Ae. aegypti are not endemic to that country (Ministry of Heath, Wellington, New Zealand, http://www.moh.govt.nz/notebook /nbbooks.nsf/0/E3EB410791DF9F974C2565D7000E22AE/ $file/mosq2.pdf), although imported Ae. albopictus has been detected in the Port of Auckland (252). It has been suggested that Aedes notoscriptus, which is a competent vector of CHIKV (253) and a vector of RRV (Alphavirus) in Australia (254), could be a potential vector of ZIKV in New Zealand (255). This mosquito is present in Province Wellington (256). Ae. notoscriptus is also a competent experimental vector of DENV and Japanese encephalitis virus (JEV) (153).
In 2007, two cases of Zika fever imported from Yap State were reported in Guam (Western Pacific); those cases were not confirmed (257). The last reported DENV outbreak in Guam occurred in 1944, before Ae. aegypti was eliminated. In April 1995, an entomological survey found no Ae. aegypti mosquitoes on the island, but Ae. albopictus mosquitoes were abundant (258).
ZIKV infections have been reported in Australia in travelers returning from Jakarta and Bali (Indonesia) in 2013 and 2015 (259, 260), respectively, and the Cook Islands in 2014 (261) and 2015 (262). The Bali patient developed confirmed Zika fever 7 days after a monkey bite, but the mode of contamination was not confirmed because he had been exposed to mosquitoes. A total of 12 imported cases were reported as of June 2014 (153). The risk of ZIKV emergence in Australia depends on the region. Seven of the 12 imported cases were recorded in Queensland, where Ae. aegypti is found (Queensland and Torres Strait) and regular DENV outbreaks are reported (263). There is concern that Ae. albopictus, which is already present in the Torres Strait (264), may become established in Australia’s mainland (153).
The Disease Burden of ZIKV Infections
A number of factors can contribute to underestimation of the disease burden of Zika fever. Virologic confirmation by isolation of ZIKV or its RNA from humans, vectors, or hosts is limited to countries with access to cell culture and/or molecular technologies. Many of the countries at risk for ZIKV infection lack adequate laboratory facilities to perform ZIKV detection. When laboratory tools are not locally available, samples should be sent to a reference laboratory but shipment of frozen samples in dry ice from remote areas is often difficult. Even if molecular tools are available, ZIKV is not on the list of arboviruses routinely tested for. ZIKV generally causes mild disease or asymptomatic infections, and patients may not seek medical care. In addition, the disease occurs in countries where people have no or poor access to medical facilities or in which medical facilities are lacking. Only recently, seven cases of acute ZIKV infection in residents of different provinces of Thailand were confirmed by molecular testing or serology, suggesting that ZIKV is widespread throughout Thailand (265). Before that report, only imported cases in travelers returning from Thailand were reported (213, 241, 244). In June 2015, a case of Zika fever imported from the Maldives was reported in Finland, suggesting that ZIKV has circulated silently in that tourist country (266).
The incidence of Zika fever can also be overestimated. False positive molecular test and serology results can occur. Cross-reactions with other flaviviruses can overestimate the prevalence of ZIKV infections in areas where flaviviruses are endemic.
Globally, however, the real incidence, prevalence, and geographic distribution of ZIKV are likely underestimated (267). In the Pacific, some countries report cases of “acute fever and rash” (250) but these infections are not investigated and the pathogens are not identified. In Brazil, health authorities initially reported an outbreak of 6,000 cases of “exanthematic disease,” but ZIKV was detected in only a few patients, so the number of actual infections is unknown. In June 2015, the government reported 40,000 cases of infection with 24,000 suspected cases of Zika fever but in the absence of routine laboratory testing, the true number of infections is unknown (176). Like dengue in both the Pacific and the Americas, it is thought that ZIKV circulates silently in some areas without being detected (267). The situation is certainly the same in countries in Asia and Africa.
CLASSIFICATION AND PHYLOGENY OF ZIKV
ZIKV is placed in the clade X mosquito-borne Flavivirus cluster, along with SPOV (47). These results, based on partial sequencing of the gene for nonstructural protein 5 (NS5), were confirmed by sequencing the complete coding region of the NS5-encoding gene (135). The full genome of ZIKV (the ZIKV MR 766 prototype strain) was entirely sequenced for the first time in 2007 (268). The full sequences of other ZIKV strains from Brazil, Cambodia, the Central African Republic, French Polynesia, Guatemala, Malaysia, Nigeria, Puerto Rico, Senegal, Thailand, and Yap State are available in GenBank (http://www.ncbi.nlm.nih.gov/GenBank/) (12, 135, 146, 269).
The genome structures of the ZIKV MR 766 prototype strain and the French Polynesian H/PF/2013 strain are detailed in Table 2. ZIKV, like other flaviviruses, is a single-stranded, positive-sense RNA virus with a genome of 10,794 kb (268, 270) with two flanking noncoding regions (5' NCR and 3' NCR). The open reading frame (ORF) encodes a polyprotein with three structural proteins, i.e., capsid (C), premembrane/membrane (PrM), and envelope (E), and seven nonstructural proteins, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 (3, 216, 268).
The 2007 Yap strain, the French Polynesian H/PF/2013 epidemic strain, and three strains of ZIKV from Senegal had a glycosylation motif at position 154 of the envelope (135, 216). This glycoprotein motif has been described in several flaviviruses but not in the ZIKV MR 766 prototype strain. This glycosylation site has been associated with an increase in virulence (135, 216). It has been suggested, but not proven, that the ZIKV MR 766 prototype strain lost this glycosylation motif during extensive mouse brain passages. Evidence of passage-associated changes in potential glycosylation sites was obtained by sequencing ZIKV MR 766 prototype strains with different passage histories (271). The loss of this glycosylation motif by WNV (272) or Kunjin virus (273) after several passages has also been reported.
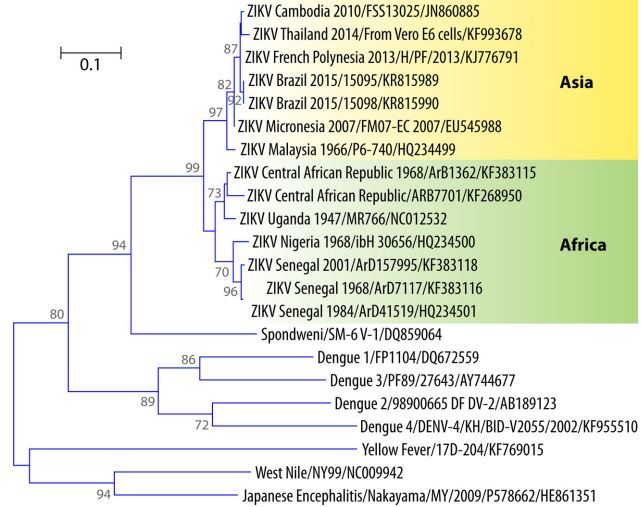
The first phylogenetic study of ZIKV was conducted by Lanciotti et al. after the Yap State outbreak (135). Sequencing of the complete coding region of the NS5-encoding gene revealed three different ZIKV lineages or subclades: East African (prototype Uganda strain), West African (Senegal strains), and Asian (ZIKV 2007 Yap strain). The Asian lineage diverged from a common ancestor that moved in Southeast Asia and the Pacific (135).
On the basis of the full genome sequences of the ORFs, Haddow et al. described two major ZIKV lineages, African (Nigeria, Senegal, and Uganda strains) and Asian (Malaysia 1966, Yap State 2007, and Cambodia 2010), suggesting that ZIKV was introduced into Yap State from Southeast Asia (271) and that ZIKV has circulated in Southeast Asia since at least the 1960s.
Faye et al. sequenced the E- and NS5-encoding genes of 43 ZIKV strains isolated from 1947 to 2007 in Africa, Asia, and Oceania (3). This phylogenetic study suggested that African strains were arranged into two groups, the ZIKV MR 766 prototype strain Uganda cluster and the Nigeria cluster; the ZIKV 2007 Micronesian and Malaysian strains constituted the Asian clade. Viruses isolated in Ivory Coast and Senegal were found in both of the African clusters, suggesting that strains belonging to both clusters cocirculated in West Africa. The authors suggested that ZIKV emerged in Uganda (East Africa) around 1920 and moved to West Africa. Two independent introductions from East Africa to West Africa occurred, the first one from Uganda to Ivory Coast and Senegal around 1935 to 1940 related to the MR 766 prototype strain cluster and the second one from Uganda to Nigeria and Central Africa around 1935 with subsequent dispersion to Senegal. The Ivory Coast and Burkina Faso viruses were related to the Nigerian cluster. ZIKV probably moved to Asia in the 1940s and then spread throughout the region, forming the Asian lineage (3). The results corroborated the existence of the Asian and African lineages. This was confirmed by Grard et al., who sequenced the E- and NS3-encoding genes (14).
Faye et al. suggested that ZIKV potentially experienced several recombination events in nature (3). However, recombination in members of the Flavivirus genus has not been demonstrated in nature or experimentally; recombinations were detected only by utilizing computationally demanding phylogenetic analyses. The most convincing evidence of Flavivirus recombination was described in a DENV serotype 1 (DENV-1)-infected patient in New Caledonia (274). These data should be interpreted with caution and confirmed with further studies.
The percent identity of the entire coding region of the ZIKV 2007 Yap strain with that of the prototype ZIKV MR 766 prototype strain was 88.9% (96.5% at the amino acid level) (135).
FIG 6 Phylogenetic tree of ZIKV showing the African and Asian lineages, including the strains that recently emerged in the Pacific and Brazil.
According to partial sequencing of the M/E-encoding gene, the French Polynesian strain was closer to the strain isolated in Cambodia in 2010 than to the ZIKV 2007 Yap strain (17), both of which are in the Asian lineage (275). Ion torrent sequencing analyses of two isolates collected during the French Polynesian outbreak evidenced genomic microevolution during the epidemic (146).
In the Americas, ZIKV sequences were available from Brazil (27, 28, 276) Colombia (196), Puerto Rico (269), and Guatemala (269). All of them showed more than 99% nucleotide identity with the French Polynesian strains (269). These American strains can constitute a “Western Hemisphere group” within the Asian genotype (269). The NS5-encoding gene of the strain isolated on Easter Island also showed 99.8% identity at the nucleotide level with the French Polynesian strain (149).
Collectively, epidemiologic and sequence data support the hypothesis that the epidemic strains of ZIKV emerged via genetic change in the Asian lineage virus. Most likely, two such events occurred, one in Yap State and the other in French Polynesia. The latter strain had greater virulence and was introduced to Brazil in 2015 and from Brazil to other American countries. An alternate hypothesis is that the emergence of severe disease associated with ZIKV was a function of incidence. Low-frequency events such as GBS and microcephaly might only be recognized during an epidemic with large numbers of cases, such as those seen in French Polynesia (>30,000 cases) and Brazil (>1,000,000 cases). Thus, with only 14 human cases recognized prior to the Yap State epidemic, the possibility cannot be excluded that the ancestral strains of ZIKV were capable of causing severe complications that went unrecognized because the number of human cases was too small.
Interestingly, the partial sequence of the NS5-encoding gene of the strain isolated from a traveler returning from the Maldives in June 2015 was identical to those of the strains from French Polynesia, Brazil, and Easter Island (266), suggesting a probable introduction from the Pacific or Brazil. Phylogenetic data are not yet available for the strain that emerged in Cape Verde in late 2015. A phylogenetic tree based on the partial sequence of the E-encoding genes of ZIKV and other flaviviruses is shown in Fig. 6.
ECOLOGY
Host/Reservoir
Nonhuman primates . ZIKV antibodies have been detected in different monkey species in Africa and Asia (Table 3). Wolfe et al. (277) and Kilbourn et al. (278) tested human and monkey serum samples; as ZIKV seroprevalence was higher in humans (44.1%) than in orangutans (8.5%), they concluded that orangutans may have been infected with ZIKV from a human reservoir or from recently established sylvatic cycles (277, 278) and that nonhuman primates may be reservoir hosts of ZIKV in Asia. Epizootics of ZIKV in monkeys were reported in Uganda in the Entebbe Peninsula in 1947, 1948, 1956, 1962, 1963, 1969, and 1970 (13, 126). Another epizootic of ZIKV was reported in the Kedougou region of Senegal in 1973, with Aedes luteocephalus and Aedes furcifer- taylori as the main vectors (279). However, animal serosurvey results must be interpreted with caution because of cross-reactivity (126); animal and human studies were often conducted at the same time with the same methods and reagents (280).
Other species . Serosurvey studies detected antibodies to ZIKV in bats (281), goats (121), rodents (Tatera indica, Meriones hurrianae, and Bandicota bengalensis) (121), and sheep (121). These data may indicate cross-reactivity with other flaviviruses but suggest that there is no clear association between ZIKV and a particular animal species . However, ZIKV has never been isolated from nonprimates, so it is not clear whether other species can act as reservoir hosts (282).
TABLE 3 Animal studies on antibody detection and isolation of ZIKV
a MI, mouse inoculation. b Kisubi and Bwamba. c HA, hemagglutination assay. d ZIKV MR prototype strain.
In Africa, ZIKV is probably maintained in a sylvatic cycle involving nonhuman primates and mosquitoes, with cyclic epizootics in monkeys. In areas without nonhuman primates, such as Yap State and French Polynesia (271), ZIKV is probably maintained in a human-mosquito-human cycle, suggesting that the virus has adapted to humans as a reservoir host. Since animal studies have not been conducted in these islands, however, the occurrence of another reservoir host cannot be excluded. Animal ZIKV sero- surey results are presented in Table 3.
Vectors and Vector-Borne Transmission
ZIKV was first isolated from Ae. Africanus in 1948 (1). Subsequent isolates of ZIKV from this species included 2 strains from the Lunyo Forest (9) and 12 strains from the Zika Forest of Uganda (10). Other arboviruses (Ntaya virus, YFV, Rift Valley fever virus, and CHIKV) have also been isolated from Ae. africanus (11). This mosquito prefers monkeys to humans (283) but also feeds on rodent, avian, and reptilian species (10).
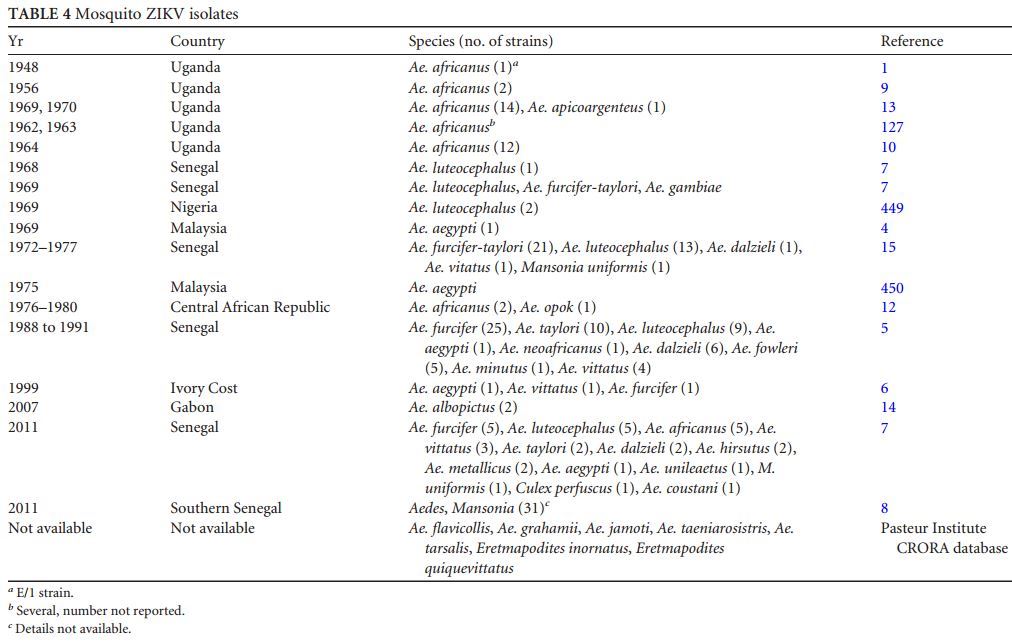
The first isolate of ZIKV in Asia was obtained from Ae. aegypti in Malaysia in 1966; it was the first isolate of ZIKV from a mosquito other than Ae. africanus (4). ZIKV was isolated from a male Aedes furcifer mosquito (7), suggesting possible vertical transmission , which could be an important mechanism of ZIKV maintenance in nature. The seasonal distribution of the ZIKV infection rate in mosquitoes in Senegal showed two peaks of amplification, in June and between September and December; 31 strains of ZIKV were isolated from mosquitoes (7). ZIKV mosquito isolates are presented in Table 4.
The isolation of a virus from a mosquito is not evidence that it is a vector of the virus. To demonstrate that a mosquito is a vector, it must be shown to be capable of transmission (108). The first ZIKV vector competence study was conducted in 1956 with Ae. aegypti (284). Transmission of ZIKV by Ae. aegypti using a mouse skin membrane and heparin-treated blood to infect mosquitoes was successful. The ZIKV loads in the mosquitoes were measured by determining the mouse 50% lethal doses (LD50) by the method of Reed and Muench (285). ZIKV was not detectable on days 5 and 10, but by days 15 and 20, the ZIKV load had increased to 103.4 and 105.6 mouse LD50, respectively. It remained constant at approximately 105.0 mouse LD50 from day 25 to day 60, suggesting that Ae. aegypti is capable of transmitting ZIKV to a susceptible host for 10 weeks . A rhesus monkey was successfully infected by the bite of three infected Ae. aegypti mosquitoes. The extrinsic incubation period of ZIKV (the time between infection of the vector and when it becomes able to transmit the virus) was about 15 days. In a comparison of YFV and ZIKV vector competence, the extrinsic incubation period was shorter and transmission was more efficient with ZIKV (286). The authors suggested that these data could explain, in part, the lower frequency of YFV epizootics observed in eastern Senegal.
The geographic variation in the oral susceptibility of mosquitoes of the same species to different viruses is well documented (287-289). The susceptibility of an Asian strain (Singapore) of Ae. aegypti to the MR 766 prototype strain of ZIKV was investigated under conditions that mimicked the local climate (290).
TABLE 4 Mosquito ZIKV isolates
Mosquitoes were infected orally. ZIKV was detected in salivary glands from day 5 in 62 % of the infected mosquitoes and in all of the infected mosquitoes on days 10 and 14, demonstrating that Singapore’s Ae. aegypti was highly capable of transmitting ZIKV. The decrease in the midgut viral titer observed at day 14 was consistent with studies conducted with DENV and WNV. The ZIKV infection level was found to be higher in saliva than in the midgut, suggesting that viral dissemination and amplification within the salivary glands or other organs and tissues are more important than dissemination from the midgut. However, Ae. aegypti strains from Kedougou and Dakar (Senegal) were susceptible to oral infection but not competent to transmit ZIKV (291), showing that the competence of Ae. aegypti to transmit ZIKV, like DENV, depends on the mosquito strain (288). However, Ae. aegypti, with poor competence but high density, has been shown to be a vector of arbovirus outbreaks (292).
Ae. albopictus has been shown to experimentally transmit 27 arboviruses , including ZIKV (293, 294). A Singapore strain infected orally with the ZIKV MR 766 prototype strain had ZIKV in its salivary glands by day 7 and thus was potentially infectious (294). Ae. luteocephalus and Aedes vittatus were susceptible to ZIKV infection, but only a small proportion of them were able to transmit the virus (291).
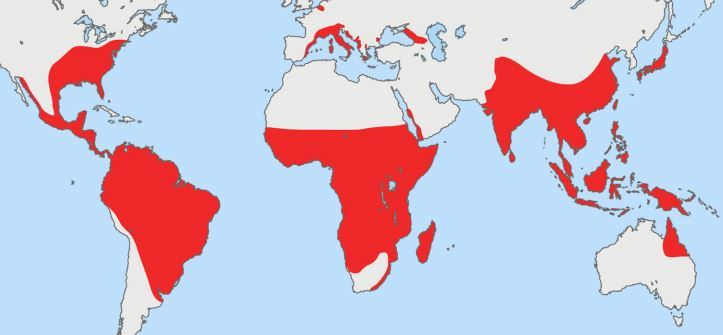
An entomological study conducted in Yap State identified 12 mosquito species belonging to four genera. The predominant species were Ae. hensilii (41.2%) and Culex quinquefasciatus (28.1%); no virus was found in any field-collected mosquitoes (16, 295). Because of its abundance in Yap State and the fact that it was the likely vector of DENV there (132), Ae. hensilii was the most plausible vector of ZIKV in Yap State (16). Experimental studies were conducted with Ae. hensilii collected in Yap State with the ZIKV MR 766 prototype strain (295). Up to 86.1% of the mosquitoes receiving a high-level dose of ZIKV (5.9 log10 PFU/ml) became infected, but only 22.6% of them developed a disseminated infection. Those mosquitoes feeding on the lowest-level dose (4.9 log10 PFU/ml) of ZIKV were resistant to infection (7.1% infection). Ae. hensilii has a limited distribution in the Pacific islands, i.e., Yap, Palau, and Chuuk (Federated States of Micronesia). Ae. hensilii is not present in the other Pacific islands where ZIKV has spread since 2013.
Two potential vectors of ZIKV are present in French Polynesia: Ae. aegypti, the main vector of DENV (296, 297), and Aedes polynesiensis, the main vector of lymphatic filariasis in this country (298, 299); Ae. hensilii and Ae. albopictus are not present. During the French Polynesian outbreak, 238 female Ae. polynesiensis, 286 female C. quinquefasciatus, and 2,039 female Ae. aegypti mosquitoes were collected and tested for ZIKV infection by RT-PCR. ZIKV RNA was detected in only one Ae. aegypti mosquito pool (V. Richard, Institut Louis Malarde, Tahiti, French Polynesia, personal communication). However, experimental studies showed the French Polynesian strain of Ae. aegypti to be able to replicate the French Polynesian ZIKV strain. By day 9 postinfection, 75% of the infected mosquitoes showed viral dissemination, although salivary gland infection remained low (8%) (300).
In New Caledonia, ZIKV was probably transmitted by Ae. aegypti, which is the vector of CHIKV (301) and DENV (302); Ae. polynesiensis, Ae. albopictus, and Ae. hensilii are not present.
Experience with ZIKV in the Pacific confirmed that ZIKV can be transmitted by different vectors during outbreaks, i.e., by Ae. hensilii in Yap State, Ae. aegypti in New Caledonia, and Ae. aegypti and/or Ae. polynesiensis in French Polynesia. In Gabon, Ae. albopictus, introduced into an environment where the Ae. aegypti level was low, was the vector of ZIKV (14). Together, these data indicate that, as for the lack of a clear pattern of preference for animal species, there is a lack of clear preference of ZIKV for mosquito vector species (3). The competence of American strains of Ae. aegypti and Ae. albopictus to transmit ZIKV is unknown, but epidemiological and experimental studies have shown that both species are well adapted to transmit DENV and CHIKV (191).
Non-Vector-Borne Transmission
Laboratory contamination. A laboratory staff member developed a febrile illness after yellow fever vaccination (17D vaccine), but ZIKV was isolated from blood taken the first day of illness. The infection was believed to be laboratory acquired (303).
Sexual transmission . Four reports suggest the possible sexual transmission of ZIKV. In 2008, an American scientist conducting mosquito field work in Senegal became ill with common symptoms of ZIKV infection after returning to the United States. He also had prostatitis and hematospermia. His wife, who had no history of travel outside the United States since 2007, had sexual intercourse with her husband the day after he returned home. She subsequently developed a Zika fever-like illness, suggesting transmission by sexual intercourse. Both patients were confirmed as ZIKV infection by serological testing (HI, plaque reduction neutralization test [PRNT], and CF) (232). Also, in December 2013, during the French Polynesian outbreak, a 44-year-old man sought medical care for hematospermia. The patient presented no signs of urinary tract infection, prostatitis, urethritis, or cystitis, and he reported no recent close contact with persons with acute ZIKV infections. Blood and semen samples were collected; ZIKV RNA was detectedbyRT-PCR, and ZIKV was isolated by inoculation of semen samples onto Vero cells. A second set of samples was collected; ZIKV was detected in semen and urine but not in blood. The detection of ZIKV in semen while it was not detected in blood collected at the same time suggested viral replication in the genital tract. Also, ZIKV was recently isolated from the convalescent- phase semen of a patient, but his serum and urine were negative (480) and a case of Zika fever transmitted by sexual contact has been reported in Texas (235,304). These results confirmed that ZIKV can be transmitted by sexual intercourse (305) and is a potentially sexually transmitted virus (306). The ECDC recommends deferral of semen donation for 28 days after returning from areas where ZIKV is endemic (31). Although the main mode of transmission of Zika fever is thought to be via mosquito bite, the low viremia observed in patients and the rapid spread within and among countries in a region like the Americas suggest other modes of transmission. The evidence of sexual transmission suggests a mode of interhuman transmission that could contribute to its rapid spread.
Maternofetal transmission . Perinatal transmission has already been reported for other flaviviruses such as DENV (66, 67) and WNV (70, 71), as well as alphaviruses such as CHIKV (68, 69), so it should not be surprising if it occurs with ZIKV.
Two cases of perinatal transmission of ZIKV were reported during the French Polynesian outbreak (307). ZIKV RNA was detected in serum samples from both mothers and infants and in both mothers’ milk. One of the infants remained asymptomatic, while the other had a maculopapular rash with thrombocytopenia. Both the mothers and the infants recovered uneventfully. Even though no infective ZIKV particles were detected in breast milk, the possibility of ZIKV transmission by breastfeeding must be considered. Given the severe neonatal complications reported after CHIKV (69) and DENV (66, 67) infections, authors recommended close monitoring of perinatal ZIKV infections even before the description of severe complications in Brazil. Maternofetal transmission was confirmed in Brazil in pregnant women who gave birth to neonates with severe malformations; ZIKV RNA was detected in amniotic fluid and blood and tissue samples from microcephalic newborns (31, 31, 37, 276, 308). The French Polynesian data suggested perinatal transmission; the Brazilian cases suggest that it can also occur transplacentally during pregnancy, causing severe malformations.
Transfusion-transmitted infections . Arbovirus transmission by transfusion of blood products has been documented for DENV (75, 76), WNV (77, 309), and RRV (78). Given its epidemiology, the possibility of ZIKV transmission via transfusion should be considered as well (310, 311). To prevent potential ZIKV transmission by transfusion, a specific nucleic acid testing protocol was implemented during the French Polynesian ZIKV outbreak (312). From November 2013 to February 2014,42 (2.8%) of 1,505 blood donors tested were confirmed positive for ZIKV RNA; all of them were asymptomatic at the time of blood donation. Eleven of the 42 blood donors developed a “Zika fever-like syndrome” within 3 to 10 days after blood donation (313). No transfusion-transmitted Zika fevers were documented during this outbreak, but the possibility that asymptomatic posttransfusion infection occurred cannot be ruled out. Unfortunately, blood samples collected within the first week after transfusion were not available. These results suggested that ZIKV can be transmitted by blood transfusion and that ZIKV nucleic acid testing can prevent the transmission of ZIKV by blood transfusion. In areas with vectors competent for ZIKV transmission, epidemic preparedness plans should include sustainability of the blood supply (141).
In addition to nucleic acid testing of blood donors, prevention of posttransfusion Zika fever can be performed by pathogen inactivation in blood products (314). Pathogen inactivation was of particular interest in the context of the cocirculation of several arboviruses during the ZIKV outbreak in French Polynesia and can be of great interest in the Americas (315). Arboviruses in blood products, including CHIKV, WNV, and DENV, can be inactivated by treatment with amotosalen and UVA illumination (316). The efficacy of this blood component treatment was demonstrated for ZIKV; amotosalen combined with UVA light inactivated ZIKV in fresh frozen plasma (6.57 log10 by infectivity assay and 10.25 log10 by RT-PCR assay) (317). The ECDC recommends deferral of blood donation by people returning from areas with active ZIKV circulation (for 14 days, the same as for dengue), deferral for 28 days after cessation of symptoms for blood donors with confirmed ZIKV infection, and implementation of pathogen inactivation in platelets and fresh frozen plasma in infected areas (26). They also recommend transfusion of blood products to pregnant women only after the products test negative for ZIKV. However, this requires a laboratory with the capacity to perform molecular screening of blood donors. The first case of ZIKV transmission by blood transfusion was reported in Brazil in December 2015 (206).
PATHOPHYSIOLOGY OF ZIKV INFECTIONS Mechanisms of Infection
Data on the pathogenesis of ZIKV are scarce. Human dermal fibroblasts, epidermal keratinocytes, and immature dendritic cells were found to be permissive to ZIKV infection (318). The DC- SIGN, AXL, Tyro, and TIM-1 entry/adhesion factors permit the entry of ZIKV. ZIKV replication activates an antiviral immune response and the production of type I interferon in infected cells. The formation of autophagosomes is associated with enhanced viral replication, and the induced expression of antiviral antigen clusters (RIG-1, MDA-5, and TLR3) that are able to detect the presence of pathogen-associated molecular patterns was observed after infection of skin fibroblasts. ZIKV infection induced an autophagous program confirmed by the presence of characteristic autophagosome-like vesicles in the infected fibroblasts (318). T cells are activated during the acute phase of Zika fever (Th1, Th2, Th9, and Th17) (319).
Replicative Cycle
The replicative cycle has been poorly studied. The detection of virus-specific antigens by indirect immunofluorescence in the nuclei of infected Vero cells was reported (320). Before that study, the replication cycle of arboviruses was thought to be exclusively cytoplasmic (44).
Animal Studies
In the initial rhesus monkey experiments (1), only monkey 766 developed slight pyrexia and circulating virus was demonstrated in its serum on day 3 of fever. Rhesus monkeys inoculated subcutaneously developed no signs of pyrexia but developed antibodies within 2 to 3 weeks after infection (117).
In mice inoculated intracerebrally, the only organ that contains demonstrable quantities of virus at the onset of illness was the brain (117). Cotton rats, guinea pigs, and rabbits also inoculated intracerebrally show no signs of infection, but rabbits develop antibodies to ZIKV by 21 days postinfection. The changes described by Dick (117) in mice sacrificed on the first day of signs of infection were confined to the central nervous system. Other lesions that have been demonstrated in mice are skeletal myositis, myocarditis, and lung edema in those with marked myocarditis (9). Histopathologic examination of infected mouse brains showed neuronal degeneration, cellular infiltration of the cords, and inclusion bodies of Cowdry type A in damaged nerve cells. Neuronal degeneration was most intense in the hippocampus region (9). Other lesions in Ammon’s horn were reported in mice inoculated intracerebrally (321). Neurotropism of ZIKV in mice was demonstrated after intracerebral inoculation, but it was not demonstrated that other modes of transmission can lead to central nervous system damage. The marked neurotropism of ZIKV in mice was in contrast to its lack of neurotropism in monkeys, cotton rats, guinea pigs, and rabbits (117). Experimental animal infections can be performed by the intracerebral, intraperitoneal, and subcutaneous routes; adult mice can also be infected by intranasal inoculation (117).
Cross Protection from ZIKV and Other Arboviruses
Several publications have shown that animals experimentally infected with an immunologically related arbovirus are protected to some degree against fatal infection (322-324). Vervet monkeys immunized with ZIKV had detectable viremia when challenged with YFV, but none of them died and the viremia titer was lower than in naive monkeys (325). ZIKV immunization of rhesus monkeys altered the severity of hepatic lesions due to YFV and prolonged survival, while no sparing effect was noted in other organ systems (326). Of the monkeys collected during the YFV epizootic in the Zika Forest in 1971, 40% were immune to YFV but had no antibodies to ZIKV, suggesting that the two viruses may not coexist in the same ecosystem (327). The hypothesis that ZIKV interferes with subsequent YFV viremia and immunity (325) was not supported by the intensive YFV epizootic that occurred 18 months after a ZIKV epizootic in the Zika Forest of Uganda in 1970 (13).
The prevalence of antibodies to at least one DENV serotype was 80.3% in donor blood collected from 2011 to 2013 in French Polynesia before the ZIKV outbreak, demonstrating that high DENV sero- prevalence does not protect against large ZIKV epidemics such as that which occurred in Yap State (132,133) and in French Polynesia (137, 138). Moreover, coinfections with other arboviruses were reported, i.e., DENV-ZIKV during the French Polynesian (Musso, unpublished data, 2014) and New Caledonian (144) ZIKV outbreaks and DENV-CHIKV-ZIKV in Colombia (328). These data show that DENV infection does not protect against ZIKV infection.
LABORATORY DIAGNOSIS OF ZIKA FEVER
Laboratory Safety
Depending on the country, ZIKV maybe classified as a level 2 or 3 pathogen. The United Kingdom has classified ZIKV as a level 3 pathogen requiring a biosafety level 3 laboratory according to the MIS208-HSE approved list of biological agents (Health and Safety Executive, http://www.hse.gov.uk/pubns/misc208.pdf); while the CDC and the National Institutes of Health in the United States (329) and the World Health Organization (330) have classified it as a level 2 pathogen requiring only a biosafety level 2 laboratory. ZIKV is killed by potassium permanganate at 0.5%, 24 h of contact with ether, and temperatures above 60°C but is not inactivated by 10% ethanol (117).
Clinical Laboratory Testing
Several blood disorders, such as leucopenia (28,144,145,217,221, 230, 247, 259), the presence of activated lymphocytes (144, 145, 221, 259), thrombocytopenia (144, 145, 221, 222, 241, 242, 247, 307), albuminemia (2), the presence of bile pigment in urine (2), and increased transaminase levels (144, 217), have been reported, but their incidence is unknown and they are common in many viral infections. Nevertheless, a standard complete blood count is recommended for all suspected cases of Zika fever for differential diagnosis.
Virus Detection
Antigen detection . Immunohistochemistry analysis with monoclonal antibodies (320) and PCR analysis (318) can be used to detect ZIKV antigen in autopsy tissues. Acute-phase diagnosis of dengue can be performed by the detection of NS1 in blood (331, 332), but this test is not yet available for ZIKV.
Culture. Isolation of ZIKV from monkey serum samples and Ae. africanus mosquitoes was first performed by mouse brain inoculation (1). Subsequent isolation methods used include inoculation of chicken embryo yolk sacs, allantoic sacs, and chorioallantoic membrane, as well as cell cultures (333-335). ZIKV was titrated in parallel in suckling mice, in adult mice, and with 11 cell culture systems. Vero, rhesus monkey kidney (LLC-MK2), and pig kidney (PS-C1) cells were more sensitive than suckling and adult mice (334, 335). ZIKV was successfully cultured by intrathoracic inoculation of Toxorhynchites speldens and C6/C36 mosquito cells (336). ZIKV has been successfully cultured from human blood (17), semen (305), and urine (241). While it has not been isolated from breast milk, ithasbeen detected by RT-PCR (307). Isolation of viruses is of particular importance to determine the phenotypic characters of the virus (337). If infectious viruses are not available, it is not possible to perform some serological tests such as cross neutralization assays or vector competence tests.
Molecular detection . (i) Molecular detection of ZIKV RNA. As flaviviruses are RNA viruses, their amplification requires two steps, RT of genomic RNA in single-stranded DNA (cDNA), followed by conversion to double-stranded DNA and amplification of the DNA; these two steps can be performed in the same reaction (338). Real-time PCR has revolutionized PCR amplification. It combines PCR amplification with a fluorescent probe and detection of the amplified product in the same reaction. This method is faster than a conventional PCR (339).
Two strategies can be used for molecular detection of ZIKV, i.e., detection of Flavivirus RNA, which requires additional testing in order to identify which Flavivirus has been amplified, and detection of specific ZIKV RNA. Molecular detection of Flavivirus RNA uses “consensus primers” designed in regions of the Flavivirus genome that possess a high degree of sequence conservation. RT-PCR assays with “consensus primers” allows the detection of new flaviviruses or variants of known flaviviruses, but they sometimes lack sensitivity. For example, Flavivirus RT-PCR failed to amplify ZIKV RNA from the serum of an infected patient, while ZIKV RNA was detected with primers specific for ZIKV (230). Most protocols target the terminal portion of the NS5-encoding gene or the 3' NCR of the Flavivirus genome because of highly conserved regions in this part of the genome (340). ZIKV was successfully detected with Flavivirus RT-PCR assays targeting the E-encoding gene (341), the NS1-encoding gene (342), the NS3- encoding gene (343), and the NS5-encoding gene (47, 344-347). After detection of Flavivirus RNA, identification to the species level requires additional testing. Several methods can be used, i.e., RT-PCR with species-specific primers (but specific primer-probe sets and positive controls are required for all suspected flavivi- ruses); cDNA amplification, followed by restriction enzyme digestion (341); ELISA to detect amplified, digoxigenin-modified DNA (348); hybridization (345); and nucleic acid sequencing. Sequencing is now the method of choice for identification to the species level because it is available in routine practice in most molecular laboratories. In Yap State (135) and in Canadian (241) and Australian (259, 260) travelers returning from areas where ZIKV is endemic and for detection of autochthonous infections in Cambodia (349), a Flavivirus sequence was amplified by RT-PCR from the blood of patients, and ZIKV was identified by sequencing the Flavivirus sequence initially amplified. RT-PCR protocols using specific ZIKV primers and probes have been developed that target the E-encoding gene (350), the membrane-envelope junction (M/E-encoding gene), the partial envelope (pE)-encoding gene (135), and the NS5-encoding gene (8, 351). The protocol developed by Lanciotti et al. was designed to detect the ZIKV 2007 Yap strain. Even though RT-PCR is very sensitive, false-negative results compared to those of culture have been reported (8).
Moreover, ZIKV RT-PCRs do not cover the genetic diversity and geographic distribution of all ZIKV strains (8). As available primers and probes have been designed on the basis of only a few full ZIKV genome sequences, new available ZIKV sequences should be rapidly deposited to ensure that the protocol can detect the circulating strain. Commercial kits for ZIKV RT-PCR are now available, but for research only; an evaluation of these tests for diagnosis has not yet been published. Primers and probes designed for ZIKV detection are presented in Table 5
Detection of ZIKV in different body fluids . Diagnosis of Zika fever usually relies on the detection of ZIKV RNA in blood during the first few days after symptom onset. Among the 748 serum samples collected during the French Polynesian outbreak (140), the mean time from symptom onset to a positive blood test was 3 days, but some patients tested positive until day 10 (Musso, unpublished), and during the Yap State outbreak, a patient tested positive on day 11. Patients can be viremic until day 10 before symptom onset, as reported in a study of asymptomatic blood donors (312). Blood ZIKV RNA loads ranged from 7.28 X 106 to 9.3 X 108 copies/ml in symptomatic patients (135, 230, 307) and from 2.5 X 103 to 8 X 106 (mean, 7.2 X 105) copies/ml in asymptomatic blood donors (317).
DENV (352), WNV (353), and ZIKV RNAs can be detected in urine. As reported in New Caledonia (145) and French Polynesia (305, 307) and in Japanese (247) and Finnish (266) travelers, ZIKV can be isolated from urine after viremia has waned to an undetectable level. These observations suggest that molecular diagnosis of ZIKV, like that of DENV and WNV, can possibly be performed with urine collected after virus clearance from blood, thus enlarging the window for ZIKV RNA detection. ZIKV RNA loads in urine ranged from 3.8 X 103 to 2.2 X 108 copies/ml (145, 305, 307).
During the French Polynesian outbreak, blood and saliva samples were collected concomitantly from patients (140). Of 182 patients, 35 (19.2%) were positive by saliva testing but negative by blood testing, while 16 (8.8%) were positive by blood testing and negative by saliva testing; the difference in the median time after symptom onset (3 days) and the frequency of symptoms of Zika fever in patients positive only by saliva or blood testing was not significant (140). The use of a saliva sample increased the rate of molecular detection of ZIKV during the acute phase of the disease but did not enlarge the window for ZIKV RNA detection and was not related to the clinical presentation of the patients. Saliva was of particular interest when blood was difficult to collect, especially from children and neonates.
In semen, ZIKV RNA loads ranged from 1.1 X 107to2.9 X 107 copies/ml (305) and in breast milk, they ranged from 2.9 X 104 to 2 X 106 copies/ml (307).
ZIKV RNA detection on filter paper . If molecular diagnosis is not locally available, case confirmation may require shipment of frozen samples to a reference laboratory, which is expensive and most of the time impossible in remote areas. Filter papers spotted with dried blood are not subject to dangerous goods regulations (354), and this can facilitate sample storage and shipment because they can be shipped at ambient temperature (355). The use of filter papers was implemented in remote Pacific countries to improve the surveillance of dengue fever (356). This protocol is now routinely used by the Institut Louis Malarde, French Polynesia, in collaboration with the WHO to confirm arbovirus outbreaks (DENV, CHIKV, and ZIKV) (21) and leptospirosis (357, 358) in the Pacific (Solomon Islands Broadcasting Corporation).
TABLE 5 Primers and probes used for ZIKV detection by RT-PCR
Using this protocol, ZIKV RNA was detected in 49 samples collected in the Cook Islands (21) and 1 sample collected in the Solomon Islands (Auckland Regional Public Health Services, http: //www.arphs.govt.nz/health-information/communicable-disease /dengue-fever-zika-chikungunya#.VeqEFv8Vipo), leading to the identification of arbovirus outbreaks in these countries.
Serological Diagnosis
ZIKV serology is usually performed by ELISA with confirmation testing by PRNT according to standard protocols (359-363). To date, there is no validated commercial serology kit for ZIKV, but kits will be available soon. ELISA is available in many laboratories. PRNT is the “gold standard” for anti-Flavivirus antibody differentiation (361) because it is relatively specific in primary Flavivirus infections (no previous infection with another Flavivirus)
(364). PRNT, however, is done only in highly specialized laboratories, is expensive, and may require regulated laboratories because of the manipulation of live viruses. New protocols using recombinant viruses (365) or reporter virus particles (363) have been developed but are not yet available for ZIKV. The arboviral serosurveys conducted in French Polynesia were done by indirect ELISA with recombinant antigens (123, 142).
The largest experience of diagnosis of ZIKV infections by serology was the testing of serum samples collected during the ZIKV outbreak in Yap State (135). Serological analysis from primary or secondary (previous infection with another Flavivirus) infected patients was performed with IgG and IgM ZIKV ELISA. Confirmation was performed by determining the reciprocal of the serum dilution reducing the number of plaques >90% (PRNT90) for ZIKV, DENVs, YFV, JEV, Murray Valley encephalitis virus, WNV, and St. Louis encephalitis virus (135). ELISA for IgM antibody against ZIKV cross-reacted with other flaviviruses but was not believed to cross-react with alphaviruses such as RRV or CHIKV (16). In primary Flavivirus infections, the IgM antibody response was specific for ZIKV, even though a limited degree of cross-reactivity with other flaviviruses was observed, and PRNT90 was highly specific. In contrast, in secondary Flavivirus-infected patients, a high degree of serologic cross-reactivity with other flaviviruses was observed with both IgM ELISA and PRNT90 (135). Serological criteria to confirm ZIKV infection during the Yap State outbreak included a positive IgM ZIKV ELISA, ZIKV PRNT90 titers of >20, and a ZIKV PRNT90/DENV PRNT90 ratio of >4 (16).
If Zika fever is suspected in a population where other flaviviruses are endemic, serological diagnosis of ZIKV is difficult to interpret because the high degree of cross-reactions in the IgM and IgG assays could lead to false-positive results. During the French Polynesian outbreak, serological diagnosis of Zika fever was not implemented because DENV-1 and DENV-3 were cocirculating and >80% of the adult population had antibodies to at least one DENV serotype (123, 142). If the risk of cross-reactions with other flaviviruses is high in adult populations with probable prior Flavivirus infection, the risk maybe low for new immigrants from areas where ZIKV is not endemic, for tourists, and for young children. All serological results should be interpreted with regard to the status of the patient. Of note, Theiler and Casals demonstrated that a secondary Flavivirus infection resulted in an increase in heterologous antibodies to other viruses of the same group (366). Moreover, a voluntary human ZIKV infection produced antibodies to ZIKV andYFV (367),but immunization with yellow fever vaccine did not produce antibodies to ZIKV (368). These results highlight the need for the confirmation of at least some cases during outbreaks by molecular and/or viral isolation.
Diagnosis of Zika Fever in Countries Where It Is Endemic
In countries with limited laboratory capacities, molecular diagnosis is not available and arbovirus diagnosis is often performed by serologic testing by IgM ELISA or rapid tests. If local laboratories use rapid tests for dengue, it is recommended to use a combined NS1 antigen and IgM antibody test to increase the sensitivity and specificity of dengue fever diagnosis (369) because NS1 antigen detection is not believed to cross-react with ZIKV. If several patients are negative by a DENV NS1 test within the first week of a “dengue like disease,” Zika fever or other arboviruses should be suspected. In this setting, the shipment of filter papers spotted with blood to reference laboratories is of great value for diagnostic confirmation. In countries with advanced laboratory capacities, an RT-PCR assay should be the first-line test. Patients presenting in the acute phase of infection with a “dengue- or chikungunya- like syndrome” or with “fever and rash” and found to be negative by specific DENV and CHIKV RT-PCR assays should be tested with a specific ZIKV RT-PCR assay. In all areas where ZIKV is known to be endemic, other arboviruses are also endemic, making serodiagnosis difficult, especially for patients with a prior Flavivirus infection.
Diagnosis of Travelers
FIG 7 Schematic flow diagram for Zika fever diagnosis . ZIKV RT-PCR is performed on blood (or on saliva if a blood sample is impossible to collect). If Flavivirus RT-PCR results are positive, sequencing is performed. ZIKV IgM serology consists of detection by ELISA or immunofluorescence, with confirmation by PRNT if the results are positive or equivocal.
For patients returning from areas with known ZIKV transmission, the challenge is to confirm ZIKV versus other endemic pathogens. In this setting, all diagnoses of “atypical dengue” in travelers re-turning from areas where ZIKV is endemic should be carefully investigated, especially if a dengue fever diagnosis relies only on serological results. If Zika fever is suspected, a specific ZIKV RT- PCR assay of acute-phase serum samples should be performed (saliva can also be tested); urine can be tested after the acute phase of the disease. Another approach is to perform a pan-Flavivirus RT-PCR assay with sequencing of the PCR product if it is positive. When molecular testing is negative, serology can be considered, but because of the cross-reactivity noted above, results should be interpreted with caution (179). Collection of paired serum samples with a 4-week interval is recommended. As a positive ZIKV IgM result is not conclusive of ZIKV infection, PRNT should be performed for confirmation. If the patient had a previous Flavivirus infection or is living in a country where flaviviruses are endemic, molecular testing or isolation is recommended as a first- line test; if serology is performed, the result should be confirmed by PRNT. A schematic flow diagram for Zika fever diagnosis derived from the recommendations of the PAHO (37, 330) and the Haut Conseil de la Sante Publique de France (http://www.hcsp.fr /explore.cgi/avisrapportsdomaine) is presented in Fig. 7. Other specific recommendations for the diagnosis of Zika fever in pregnant women and infants have been recently issued (370, 371) and will be developed in an upcoming issue of Clinical Microbiology Reviews.
CLINICAL FEATURES OF ZIKA FEVER
In tropical Africa, the Asia-Pacific region, and the Americas, infection with more than one pathogen is common and care must be exercised in ascribing a clinical diagnosis (2). In a recent study conducted in Senegal, about 50% of the patients infected with arboviruses also had malaria; three patients were coinfected with ZIKV and malaria parasites (372). The first clinical description of a patient suffering only from Zika fever was reported in 1956; it was based on a ZIKV infection experimentally induced in a human volunteer (367). The patient was a 34-year-old European male infected subcutaneously with the strain isolated in Nigeria in 1954. The first symptoms were fever and a slight headache 82 h (3.5 days) after inoculation. The headache lasted about 2 days. A rash was not recorded, and the blood count was normal. ZIKV was isolated from the blood of the patient on days 4 and 6 after infection. By HI and intracerebral mouse protection test, an increase in antibodies to both ZIKV and YFV was demonstrated from day 8 after inoculation. The patient was exposed to female Ae. aegypti mosquitoes during the acute stage of illness, but ZIKV was not recovered from them, most likely because of the low viremia titer.
FIG 8 Conjunctivitis and rash in Zika fever.
Top left photo courtesy of H. P. Mallet, Department of Health of French Polynesia; top right, bottom left, and bottom right photos by V. M. Cao-Lormeau and E. Grange, Institut Louis Malarde.
During the Yap State and French Polynesian outbreaks, the most common clinical symptoms reported were fever, rash, arthritis and/or arthralgia and/or myalgia, conjunctivitis, and fatigue (Fig. 8). Zika fever symptoms are described in Table 6. No hemorrhagic complications or hospitalizations were reported during the acute phase of illness in these outbreaks (16) (Direction de la Sante de la Polynesie Franaise, http://www.hygiene -publique.gov.pf/spip.php?article126). Dating the onset of symptoms is difficult in Zika fever because there is no abrupt clinical onset (140, 145), as opposed to dengue fever (331) and chikungunya (373). In French Polynesia, most of the patients sought medical care for a rash probably after the viremic stage. Negative ZIKV RT-PCR results do not rule out a Zika fever diagnosis because the viremic stage is short. The incubation period rangedfrom 3.5 days for the human volunteer (367) to 6 to 10 days for returning travelers and blood donors (232, 247, 312). Evolution can be biphasic; in French Polynesia, some patients sought medical care for a second episode of “Zika-like symptoms,” as was reported for the patients with ZIKV isolated from semen (275, 305). The duration of the illness is about 1 week.
The PAHO (37,179) proposed a provisional case definition of ZIKV infection based on the definition used during the French Polynesian outbreak (Direction de la Sante de la Polynesie Franaise, http://www.hygiene-publique.gov.pf/). A suspected case is a patient with a rash or an elevated body temperature (>37.2°C) and one or more of the following symptoms (not explained by other medical conditions): (i) arthralgia or myalgia, (ii) nonpurulent conjunctivitis or conjunctival hyperemia, (iii) headache or malaise. A confirmed case: is a suspected case with a positive laboratory result for the specific detection of ZIKV.
Before the French Polynesian outbreak, the seroprevalence of IgG for ZIKV was <1% in adults (123) but increased to 50% in a cohort of children 6 to 16 years old and 66% in a cohort of people 7 to 86 years old after the outbreak (142). Compared to the estimate of 11.5% of symptomatic cases in the population (18, 141), the ratio of symptomatic to asymptomatic patients was about 1:5 to 1:6. These results cannot be compared with those of the sero- survey conducted in Yap State, which detected IgM antibodies (16). However, this ratio is similar to the estimates of DENV (75, 374) and WNV infections (375). During the ZIKV outbreak, 2.8% of the blood donors tested were positive for ZIKV (312), which suggests that about 2.8% of the asymptomatic adults were viremic during the outbreak. It should be noted, however, that the ratio of asymptomatic to symptomatic DENV, and probably ZIKV, infections can vary greatly, depending on the virus strain and background immunity.
Complications
Before the French Polynesian outbreak, Zika fever was described as a mild, self-limiting, febrile illness without severe complications and a low hospitalization rate (40, 141). This description was based on a limited number of cases and one outbreak investigation, but the experience in French Polynesia changed that perception, with the description of severe neurological complications (18). With the emergence of ZIKV in Brazil, severe neonatal complications have now been reported (36).
Neurological complication in adults. During the French Polynesian outbreak, an unexpectedly high number of GBS cases was observed. The established incidence of GBS is about 1 to 3 cases per 100,000 inhabitants per year (376). In French Polynesia, the annual number of reported cases was 5, 10, 3, and 3 in 2009 to 2012, respectively (141). In December 2013, the first patient with GBS was hospitalized 7 days after presenting with a “Zika-like syndrome” (low-grade fever, myalgia, rash, and conjunctivitis) (34). During the epidemic, 42 GBS cases were reported or approximately a 20-fold higher incidence than expected (Direction de la Sante de la Polynesie Franaise, http://www.hygiene-publique.gov .pf/). All GBS patients developed neurological symptoms following a “Zika-like syndrome” episode; 74% of them were male, the median age was 42 (range, 20 to 74) years, all were natives of French Polynesia, 15 were admitted to the intensive care unit, and 9 underwent mechanical ventilation, but no deaths were reported including Flavivirus (DENV [378] and WNV [379, 380]) and Alphavirus (CHIKV [381, 382]) infections.
TABLE 6 Clinical symptoms of Zika fever
In the Americas, an increase in GBS has been reported in three countries (31). From January to July 205, Brazil reported 121 cases (377). The temporal and spatial association between the French Polynesian ZIKV outbreak and the highly unusual number of GBS cases (Fig. 9) suggested that ZIKV was the cause of GBS (18).
FIG 9 Temporal association between cases of Zika fever (blue columns) and GBS (red line) during the French Polynesian outbreak.
GBS has been reported as a complication of other arbovirus infections, in northeastern states; 62% of the patients had symptoms consistent with Zika fever preceding GBS. In El Salvador, of 22 cases investigated in December 2015, 54% also had symptoms consistent with Zika fever preceding GBS. In Venezuela, an increase of 2- to 3-fold from the national baseline has been recorded. Finally, a first case of GBS possibly associated to ZIKV infection has been reported by the French Ministry of Health on Martinique (31). Collectively, these epidemiological data reinforce the hypothesis of a relationship between ZIKV and GBS. Ocular complications in adults have been reported (383).
Neurological complications in neonates . From 2010 to 2014, the annual number of reported cases of microcephaly in Brazil ranged from 150 to 200 (384). A relationship between ZIKV and microcephaly was first suspected in Brazil in late October 2015, with an increase in cases reported in Pernambuco State, northeastern Brazil (308, 384, 385). The Brazilian Ministry of Health declared a national public health emergency on 11 November (http://paraiba.pb.gov.br/saude-discute-notificacao-de-casos-de -microcefalia-na-paraiba-nesta-sexta-feira/), and three other alerts were issued by the PAHO on 17 November (36), by the ECDC on 24 November (35), and again by the PAHO on 1 December (37). A task force was established to investigate the link between ZIKV infections during pregnancy and microcephaly (38). In the middle of December, 1,761 cases were reported in 13 states (208). As of late January 2016, Brazil has reported 3,893 cases of microcephaly since October 2015 (209). Cases were reported in 21 states and 724 municipalities (31). In early February 2016, the WHO declared a global health emergency (386).
After retrospective investigations, the French Polynesian health authorities reported 17 central nervous system malformations (including microcephaly) in newborns coinciding with the ZIKV outbreak in French Polynesia. The annual average number of central nervous system malformations in this country is about one (31,35, 37).
An etiologic relationship between ZIKV and microcephaly has not yet been firmly demonstrated (387), but new virologic and epidemiologic data favor the hypothesis of cause and effect. ZIKV RNA was detected in the amniotic fluid of pregnant women who gave birth to microcephalic newborns and in the brains of microcephalic newborns (31, 37, 276, 308, 388). These data confirm that, like TORCH viruses (toxoplasmosis, other [syphilis, varicella-zoster, parvovirus B19], rubella, cytomegalovirus, and herpesvirus) (389, 390), ZIKV is associated with severe neurological damage in newborns. However, several etiologies of microcephaly have been identified (35) and the number of microcephaly cases directly associated with ZIKV is unknown (370, 391).
The possibility cannot be excluded that microcephaly is only the tip of the iceberg and that other complications, less severe or affecting not only the brain but other organs, might occur. Other neurological, ophthalmologic, and auditory complications are now reported in neonates (38, 209, 276, 308, 371, 392). These reports will be reviewed in an upcoming issue of Clinical Microbiology Reviews.
ZIKV-related death . In addition to microcephalic neonates who died in the 24 h after death (31), three ZIKV-related deaths were reported in late November in Brazil (two adults with neurological disorders and one newborn) (37, 393). A ZIKV-infected 15-year-old female suffering from sickle cell disease died despite management in a pediatric intensive care unit (394).
Differential Diagnosis
The clinical presentation of Zika fever is not specific and can mimic diseases responsible for fever, rash, and arthralgia, especially dengue and chikungunya (271, 395). Sporadic cases of Zika fever in areas where dengue and/or chikungunya are endemic can be difficult to diagnose clinically, highlighting the importance of laboratory investigation of patients presenting with “dengue-like syndromes” and testing negative for dengue. Algorithms comparing clinical symptoms of Zika fever, dengue, and chikungunya (396) have been proposed but should be used with caution, especially when several arboviruses are cocirculating.
Treatment
There is no specific treatment or antiviral drug for ZIKV infection (179). The current treatment guidance is based on a limited body of evidence. Recommendations are the treatment of symptoms based on acetaminophen for fever and pain, an antihistaminic for pruritic rash, and drinking offluids. Treatment with acetylsalicylic acid and nonsteroidal anti-inflammatory drugs is discouraged because of the reported increased risk of hemorrhagic syndrome FIG 10 Global distribution of Ae. aegypti and Ae. Albopictus.
with other flaviviruses (Secretariat of the Pacific Community, http://www.spc.int/phs/english/publications/informaction/IA27 /Zika-outbreak-Yap-2.pdf). In the first days after symptom onset (viremic phase), patient isolation to avoid mosquito bites is recommended to prevent the infection of other persons (179).
PREVENTION OF ZIKA FEVER
There is no vaccine for ZIKV, although several are in the development phase with dengue vaccine technology. Prevention measures are therefore the same as for all Ae. aegypti-borne diseases for which there are no vaccines, including individual protection from mosquito bites and vector control. The literature on DENV prevention and control is voluminous and will not be reviewed here. See the previously published reviews on public health strategies (397-400) and mosquito bite prevention guidelines (51). The epidemiological alert for ZIKV prepared by the PAHO recommended integrated vector management and personal prevention measures (171). The spread of ZIKV highlights the need to develop new vector control strategies (401).
Specific recommendations have been issued to prevent congenital complications of ZIKV infections (37,370,402,403); these recommendations will be developed in an upcoming issue of Clinical Microbiology Reviews.
SURVEILLANCE OF ZIKA FEVER
All countries at risk for ZIKV infection, e.g., those infested with Ae. aegypti, should develop basic virologic and serologic laboratory capabilities to diagnose ZIKV. In countries without autochthonous ZIKV transmission, the PAHO recommends strengthening event-based surveillance to detect the first case (37,171,179). This would require including ZIKV testing of all patients presenting with dengue-like illness who test negative for DENV in countries with competent mosquito vectors. In countries with autochthonous transmission of ZIKV, the PAHO recommends monitoring of the trend and geographic spread of the virus within and to new countries and monitoring of genetic lineages of ZIKV (37, 171). The impact on public health should be monitored by enhancing surveillance for potential neurological and autoimmune complications, following up pregnant women and congenital malformations, and identifying risk factors associated with ZIKV infection.
THE POTENTIAL FOR ZIKV EMERGENCE
In the last 4 decades, the emergence/resurgence of epidemic arboviruses has been dramatic, affecting both humans and animals (57). There has been an increased frequency of epidemic dengue in all tropical regions of the world, and dengue hemorrhagic fever has emerged in Asia, tropical America, and the Pacific (400). Dengue is now endemic to all of the tropical areas of the world (82, 104). In the last 2 decades, WNV has emerged as a major epidemic disease of birds, horses, and humans in the Mediterranean region, Europe, and the Americas, with severe and fatal disease occurring in all of these areas (404-408). In the past decade, the status of chikungunya changed from a relatively uncommon and poorly documented disease to an emerging epidemic disease that is now a global public health concern (25, 373). There are many factors responsible for this emergence and changing epidemiology, but the principal drivers have been global population growth, urbanization, globalization, and a lack of effective vector control (57, 104).
a Reference numbers are in parentheses.
b But chikungunya is probably an older disease known as ki denga pepo (456, 457). c But dengue-like illness has been described for at least 250 years (459). d Potential risk.
e Public health problem in North America.
f In the version of this article published on 30 March 2016, “Stegomyia” was incorrectly spelled “Stemomyia.” This was changed in the version published on 9 May 2016.
TABLE 7 Common features of ZIKV and other arboviruses
The emergence of these arboviruses was associated with the description of new clinical patterns (ZIKV, CHIKV, and WNV) (18, 26,34,37,404,407,409-412) and new modes of transmission (ZIKV, CHIKV, and WNV) (68-71, 307, 312, 409, 413). There is good evidence that genetic changes in DENV, CHIKV, and WNV have been responsible for phenotypic changes influencing virulence and epidemic potential (84,85,88-94); to date, this has been suspected but not demonstrated for ZIKV (31), but it is the most likely explanation for its dramatic emergence in the past few years. The potential impact of climatic change on the spread of ZIKV in unknown (414).
ZIKV (40), like DENVs and CHIKV, has probably adapted from an ancestral forest cycle involving nonhuman primates as a vertebrate reservoir and canopy-dwelling mosquitoes as vectors to a new urban/periurban cycle involving humans and Ae. Aegypti and/or Ae. albopictus mosquitoes as vectors. Experiments are under way to confirm this adaptation of ZIKV. As widely distributed Ae. aegypti and Ae. albopictus mosquitoes are competent ZIKV vectors, the potential for the emergence of ZIKV to overlap the geographic distribution of those mosquitoes is great (Fig. 10) (ECDC, http://www.ecdc.europa.eu/en/healthtopics/vectors/) (40, 373, 415, 416). The recent coemergence of arboviruses in Pacific island countries and territories is a good example (18, 39, 40,481). Until 2007, DENV was the predominant arbovirus in the Pacific, usually with the circulation of a predominant serotype. The epidemiologic situation has since changed (39), with the cocirculation of several DENV serotypes (137) and the emergence of ZIKV in 2007 (18) and CHIKV in 2011 (25). The situation is similar in Brazil, where CHIKV emerged in 2014 (373) and ZIKV emerged in 2015 (27) in addition to numerous already endemic arboviruses, e.g., DENV and YFV (186). The epidemic in Brazil poses a high global risk, with about 10 million travelers that depart annually for international destinations; this risk will increase in August 2016, when Brazil will host the Summer Olympic Games (162).
The experience of the recent emergence of arboviruses and the facts that ZIKV has essentially the same epidemiology and mosquito vectors in urban areas as DENV and CHIKV, that it uses humans as the principal vertebrate host in urban/periurban areas, and that this human connection greatly increases the risk of global spread via infected humans to areas where mosquito control has failed (104) suggest that ZIKV will likely follow in the path of DENV and CHIKV and become a global public health problem (40). Features common to ZIKV, CHIKV, WNV, and DENV are shown in Table 7.
CONCLUSION
The first ZIKV outbreak in Yap State was unexpected and demonstrated the potential for ZIKV emergence. The second outbreak in French Polynesia was also unexpected and was associated for the first time with severe neurologic disease. From French Polynesia, ZIKV spread in the Pacific and imported cases were reported on several continents. In 2015, ZIKV emerged in the Americas and, as in French Polynesia, appears to be associated with severe neurologic disease. An outbreak has also been reported in the Cape Verde Islands (Africa). The emergence of ZIKV in areas with cocirculation of other flaviviruses will make diagnosis based on clinical and epidemiological grounds difficult and unreliable. The emergence of ZIKV in the Pacific, the Americas, and Africa underscores the potential for ZIKV to spread globally as DENV and CHIKV have done. The future of ZIKV is unpredictable, but its recent spread confirms that ZIKV is following in the path of CHIKV and DENV (40). The severe disease associated with ZIKV in French Polynesia and Brazil, however, suggests that this virus will become a very serious global public health problem.
ACKNOWLEDGMENTS
We thank all of the technicians and researchers working in the medical diagnosis laboratory and in the unit of emerging infectious disease of the Institut Louis Malarde. We thank M. Solignac of the Institut Louis Malarde for creating the figures. We thank all of the public and private health care and personal care workers who managed the French Polynesian Zika fever outbreak in 2013 and 2014. We thank the reviewers; their criticism greatly improved the manuscript.
Didier Musso is a medical doctor who graduated from the Faculty of Medicine of Aix Marseille University, Marseille, France. He has been the director of the Diagnosis Medical Laboratory and of the Unit of Emerging Infectious Diseases of the Institut Louis Malarde, Papeete, Tahiti, French Polynesia, since 2012. His research interests focus on endemic French Polynesian infectious diseases, especially arboviroses, leptospirosis, tuberculosis, and lymphatic filariasis. During the French Polynesian Zika fever outbreak, his laboratories were in charge ofidentification, diagnosis, surveillance, and research programs on ZIKV. These laboratories also received samples from collaborative countries in the Pacific area for arbovirus diagnosis and surveillance.
Duane J. Gubler has degrees in zoology/ entomology (B.S.), parasitology/entomology (M.S.), and pathobiology/tropical disease ecology (Sc.D.), the latter from the Johns Hopkins University School of Public Health in Baltimore, MD. He is currently professor and Founding Director of the Signature Research Program in Emerging Infectious Diseases, Duke-NUS Medical School, Singapore, and Chairman, Partnership for Dengue Control, Lyon, France. He served as Chair, Department of Tropical Medicine, Medical Microbiology and Pharmacology, University of Hawaii School of Medicine in Honolulu, and was the Founding Chief of the CDCDengue Branch in Puerto Rico and Director of the CDCDivision of Vector-Borne Infectious Diseases in Fort Collins. His research interests have focused on the epidemiology, prevention, and control ofvector-borne infectious diseases, with an emphasis on dengue.
Vitamin D Life pages with ZIKA in title (105 as of June 2022)
This list is automatically updated
{LIST()}
