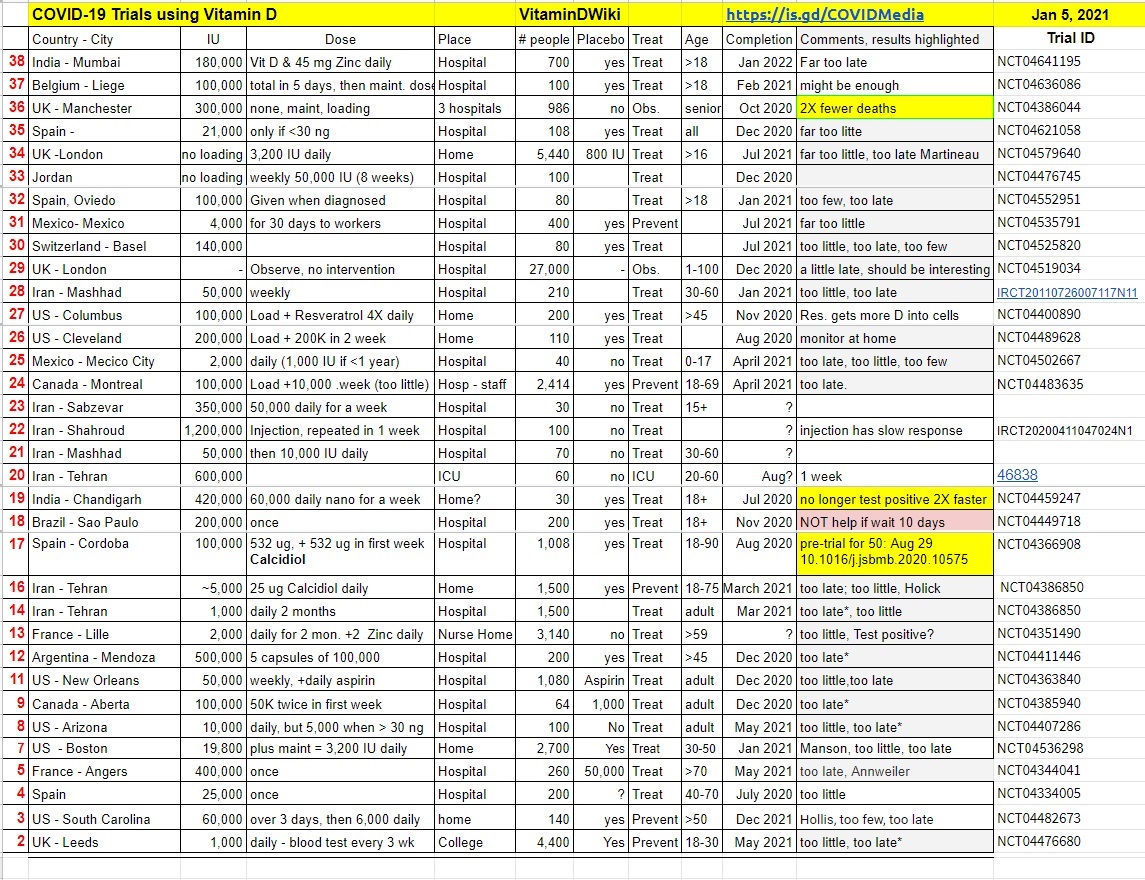
Vitamin D associated with less severe COVID-19 (Iran)
Association of vitamin D with the modulation of the disease severity in COVID-19
Virus Res. 2020 Aug 28;198148. doi: 10.1016/j.virusres.2020.198148
R Mardani 1, A Alamdary 1, S D Mousavi Nasab 2, R Gholami 3, N Ahmadi 4, A Gholami 5
Vitamin D blood measurements were taken upon entering hospital
63 confirmed COVID-19 patients and 60 COVID-19 negative controls
COVID-19 treated by Vitamin D - studies, reports, videos
- Note: Iran is running many Vitamin D RCTs for COVID-19
📄 Download the PDF from Vitamin D Life
COVID Test Positive associated with higher ACE and lower Vitamin D
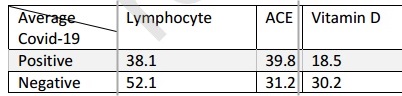
In late 2019, SARS-CoV-2 started to spread throughout the world causing the COVID-19 that has taken a considerable number of lives. Results obtained from several investigations have explained the virus origin, pathogenicity, and transmission. Similar to SARS coronavirus, the pulmonary angiotensin converting enzyme (ACE) 2 was introduced as the virus receptor for entering the cell. An increased body of epidemiological and clinical evidences has shown modulating effects of vitamin D in lung injuries through several mechanisms. Several clinical symptoms as well as molecular factors have shown to be related to the disease transmission and severity. In this study, vitamin D, ACE concentrations, and neutrophil to lymphocyte ratio (NLR) were measured in patients with confirmed COVID-19 in comparison with control group. Results demonstrated significant alterations in vitamin D and ACE levels as well as NLR in the patients' group. Contribution of those factors with the prognosis and severity of the disease has been shown.
DISCUSSIONS
By the end of 2019, COVID-19 started in Wuhan, China, and emerged rapidly as a pandemic all over the world. Quickly after its appearance, COVID-19 was detected in Iran during the winter 2020. In the present work, patients under study were individuals who had been contaminated by the SARS-CoV-2 and needed hospitalization after confirmation of the clinical COVID-19. Among them, 4 individuals (6.3% of the patients" group and 3.2% of the total) were deceased which was compliant with the death rate, previously reported for the COVID-19 [15,16]. Important changes in vitamin D and ACE concentrations as well as the NLR have shown in our study to be among important parameters associated with the severity of the COVID-19.
There is more than a century of evidence for the effects of vitamin D in remedying various pathogens' effects [17]. Extended animal studies support the regulatory effects of vitamin D on innate and adaptive immunity [18]. Vitamin D deficiency has been shown to be a risk factor in more severe courses of infection among critically ill patients. It has been observed to be inversely related to infections with various pathogens of the lower, as well as the upper respiratory tract [19]. In a 3.5 month follow-up study of a healthy cohort, a 2-fold less viral respiratory tract infections in individuals with >95 nmol/L of circulating 25(OH)VitD was observed [20].
A recent study using UK Biobank samples aimed to assess the association of blood 25(OH)VitD concentration with COVID-19 risk. The results of that study did not find important link between blood vitamin D concentrations with COVID-19 risk, nor suggested the usefulness of vitamin D measurement in clinical practice to assess the risk of COVID-19 infection [21].
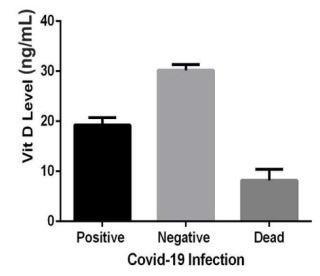
In our study, the insufficient concentrations of vitamin D were associated with the hospitalization of COVID-19 patients (Figure 1). Less than 16 ng/mL values of the serum vitamin D have been reported to be possibly associated with increased risk of sepsis in critically ill patients [22].
An optimal range for 25(OH)VitD is reported as 25-80 ng/mL, and the definition of vitamin D insufficiency is sometimes reported as <30 ng/mL. Concentrations lower than 10 ng/mL for vitamin D are reported as severe vitamin D deficiency [23].
The status ofvitamin D in the four individuals with COVID-19 who deceased in the course of this study was lower than 10 ng/mL. On admission, those individuals had severely low vitamin D levels, significantly less than both the control group and the patients" group, as depicted in Figure la.
Vitamin D is a steroid hormone that controls a broad range of metabolic and cell regulatory functions. It circulates in the blood as 25(OH) D and its concentration defines the vitamin D status of the body.
Diabetes and other comorbidities such as hypertension, obesity and ethnicity have been reported as significant predictors of morbidity and mortality in patients with COVID-19 [24]. Although it's association with ethnicity was not supported by certain other results [21].
Vitamin D is associated with many diseases through manipulating the innate and adaptive immune system pathways [25]. Multiple cells in the immune system possess the vitamin D receptor (VDR) and, are capable of converting 25(OH)VitD to l,25(OH)2VitD.
Many other cell types than kidney cells can produce l,25(OH)2VitD by the action of cytochrome p450 family member CYP27B1 , with the assistance of TLRs or alternate PRRs. Endocrine or intracrine stimulating effect of l,25(OH)2VitD on the expression of CYP27B1 enhances the epithelial cell expression of the antimicrobial peptide Cathelicidin LL-37 and beta-defensin [26, 27]. This mechanism has been shown to induce the chemotaxis of immune cells and prevent neutrophil apoptosis that increases their lifespan and consequently modulate the respiratory immune response to viral pathogens such as RSV and influenza [28, 29].
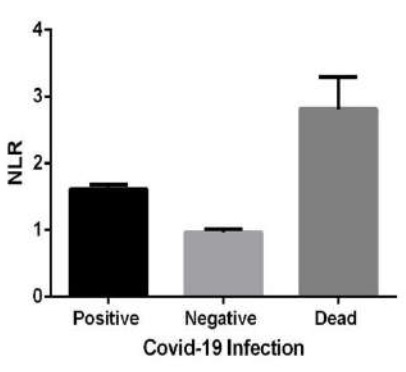
The NLR has been introduced as a useful indicator of systemic inflammation and tested as a guide for the prognosis of various diseases, including sepsis and cancer [30].
It is a routine simple measure and not costly examination in hospitals. Association of the NLR increase has also been demonstrated in ARDS and ALI [31]. Meta-analysis investigations support that NLR and LCR (lymphocyte to C-reactive protein ratio) values can help predict clinical severity in patients with COVID - 19 [32].
In the current study, significant decrease in lymphocyte along with increase in neutrophil count was demonstrated in patients (supplementary Figure 1). Consequently, we have shown the NLR increase in COVID-19 patients compared to control group, with significantly higher values in those patients for whom the disease was fatal (Table 1 and Figure 2). Considering that the blood samples of participants in this study was analyzed on admission, one possibility could be that the decreased lymphocyte count might be due to the IFN-I dependent transient lymphopenia which is observed in many viral infections [33]. It has not been demonstrated whether or not the direct viral infection through spike receptors in T-lymphocyte could contribute to lymphopenia [34]. In a study with a Time-Lymphocyte percent model in patients with COVID-19, the importance of lymphopenia, with the lymphocyte count of less than 20 percent has been demonstrated as a decisive point to predict the disease severity [35].
In our study, a significant lymphopenia was observed in COVID-19 patients, as previously reported by other studies [32, 35]. However, the blood lymphocyte in none of those deceased individuals was lower than 20 percent on admission.
Therefore, we rather suggest that the cost-effective NLR to be considered as a marker to aid complication predictions or poor prognosis in COVID-19.
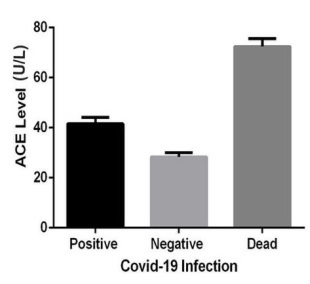
Another factor demonstrated in this study was the significant increase of circulating ACE in the COVID-19 patients (Figure IB). The renin angiotensin system (RAAS) was primarily thought to be responsible for the regulation of blood pressure and sodium and water homeostasis. However, it has been revealed that RAAS could be closely associated with the lung injury [36]. In a regular way, juxtaglomerular cells within the kidneys release renin following a blood pressure drop, which hydrolyzes circulating angiotensinogen to produce Ang I, which is then cleaved by ACE and converted to biologically active octapeptide Ang II. Ang II is the most important effector peptide of the RAAS that preferentially binds to and stimulates the Ang II type 1 receptor (AT1R), inducing vasoconstriction, inflammation, oxidative stress, and cell proliferation [37]. When metabolized by ACE2 to form Ang-(l-7), Ang II can induce the G-protein coupled MasR axis and subsequently oppose the vasoconstrictor effects of Ang II, aldosterone secretion and counteract the AT1R downstream effects. Inhibition of the Ang II signaling pathway and/or RAAS has protective effects on lung injury [38].
Clear shreds of evidence show that RAAS activation contributes to pulmonary arterial hypertension through actions of Ang II and particularly aldosterone [39]. An increase in ACE can potentially overdrive the Ang II generation and promote the detrimental effects of the AT1R classical axis. In addition to elevated ACE, we have also observed an association between increased ACE level and vitamin D insufficiency in COVID-19 patients (Figure ID). These results are in line with the fact that RAAS can provide feedback to vitamin D signaling and block the act of vitamin D as a transcription factor in renin gene suppression whereby it exerts a negative endocrine regulator activity on RAAS[40].
Lung is a major source of ACE and therefore a major site of systemic Ang II synthesis and RAAS action. It is thought that ACE2 activity is upregulated by Increased Ang II levels.
SARS coronavirus has been suggested as a predisposing factor for ARDS. RAAS components including ACE, Ang II and the Ang II type la receptor (ATla) exacerbate, while ACE2 can protect, from the disease outcomes including lung edema and impaired lung function [41]. Pulmonary ACE2 appears to regulate the balance between the levels of circulating Ang II and Ang-(l-7). Transcriptome analysis for ACE2 expression in the lungs of patients with comorbidities has shown high expressions in patients with severe COVID-19, compared to control individuals [42].
In the case of diabetes, as the expression of ACE2 depends on the progression of the disease, adverse outcomes might be reduces through patient management strategies, rigorous glucose monitoring and careful consideration of drug interactions [43].
Collectively, it seems that SARS-CoV-2 in the same way as SARS-CoV infection, could shift the balance of ACE/Ang II/AT1R axis overthe ACE2/Ang (l-7)/MasR axis in the lung, resulting in acute lung injury [44]. High expression of VDR in the lung and interaction with vitamin D can prevent lung injury through blocking the RAAS [45]. Altogether, sufficient vitamin D can have a modulating effect on the consequences of SARS-CoV-2 infection through interference with the RAAS and immune system elements functions through VDR which is a ligand-activated transcription factor,
References:
Coronaviridae Study Group of the International Committee on Taxonomy of, V., The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol, 2020. 5(4): p. 536-544.
D'Arienzoa, M. and A. Coniglio, Assessment of the SARS-CoV-2 basic reproduction number, RO, based on the early phase ofCOVID-19 outbreak in Italy. Biosafety and Health, 2020.
Muniz-Rodriguez, K., et al., Severe Acute Respiratory Syndrome Coronavirus 2 Transmission Potential, Iran, 2020. Emerg Infect Dis, 2020. 26(8).
Mardani, Rv et al., Laboratory Parameters in Detection ofCOVID-19 Patients with Positive RT-PCR; a Diagnostic Accuracy Study. Arch Acad Emerg Med, 2020. 8(1): p. e43.
Wan, Yv et al., Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies ofSARS Coronavirus. J Virol, 2020. 94(7).
Andersen, K.G., et al., The proximal origin of SARS-CoV-2. Nat Med, 2020. 26(4): p. 450- 452.
Hussain, M., et al., Structural variations in human ACE2 may influence its binding with ■S>4RS-Col/-2sp/7ceprote//i. J Med Virol, 2020.
Zheng, Y.YV et al., COVID-19 and the cardiovascular system. Nat Rev Cardiol, 2020. 17(5): p. 259-260.
Tikellis, C. and M.C. Thomas, Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int J Pept, 2012. 2012: p. 256294.
Shenoy, V., et al., The angiotensin-converting enzyme 2/angiogenesis-(l-7)/Mas axis confers cordiopuimonary protection against lung fibrosis and pulmonary hypertension. Am J RespirCrit Care Med, 2010. 182(8): p. 1065-72.
Foley, RN, P.S. Parfrey, and MJ. Sarnak, C///i/ca/ep/c/em/o/ogy o/ca厂c//o\/ascL//a厂 c//sease in chronic renal disease. Am J Kidney Dis, 1998. 32(5 Suppl 3): p. S112-9.
Martineau, A.R., et al., Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ, 2017. 356: p. i6583.
Holick, M.F., Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol, 2009. 19(2): p. 73-8.
Xie, C., et alv Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis, 2020. 93: p. 264-267.
Epidemiology Working Group for Ncip Epidemic Response, C.C.f.D.C. and Prevention, [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID- 19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi, 2020. 41(2): p. 145-151.
Team, C.C.-R., Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID- 19) -United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep, 2020. 69(12): p. 343-346.
Lang, P.O., et al., How important is vitamin D in preventing infections? Osteoporos Int, 2013. 24(5): p. 1537-53.
Hewison, M., Vitamin D and innate and adaptive immunity. Vitam Horm, 2011. 86: p. 23- 62.
Ginde, A.A., J.M. Mansbach, and C.A. Camargo, Jr., Association between serum 25- hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med, 2009. 169(4): p. 384-90.
Sabetta, J.R., et alv Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One, 2010. 5(6): p. ell088.
Hastie, C.E., et al., Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr, 2020. 14(4): p. 561-565.
Moromizato, T., et al., Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med, 2014. 42(1): p. 97-107.
Kennel, K.A., M.T. Drake, and D.L. Hurley, Vitamin D deficiency in adults: when to test and how to treat Mayo Clin Proc, 2010. 85(8): p. 752-7; quiz 757-8.
Garg, M., et al., Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North-supports vitamin D as o factor determining severity. Authors1 reply. Aliment Pharmacol Ther, 2020. 51(12): p. 1438-1439.
Prietl, B., et alv Vitamin D and immune function. Nutrients, 2013. 5(7): p. 2502-21.
Gombart, A.F., The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol, 2009. 4(9): p. 1151-65.
Adams, J.S. and M. Hewison, Extrarenal expression of the 25-hydroxyvitamin D-l- hydroxylase. Arch Biochem Biophys, 2012. 523(1): p. 95-102.
Nagaoka, I., et al., Modulation of neutrophil apoptosis by antimicrobial peptides. ISRN Microbiol, 2012. 2012: p. 345791.
Ahmed, A., et al., Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses, 2019. 11(8).
Martins, E.C., et al., Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: a case-control study. Rev Bras Ter Intensiva, 2019. 31(1): p. 64-70.
Zhang, Yv et alv Neutrophil-lymphocyte ratio as an early new marker in AIV-H7N9-infected patients: a retrospective study. Ther Clin Risk Manag, 2019. 15: p. 911-919.
Lagunas-Rangel, F.A., Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta- analysis. J Med Virol, 2〇3d.
Kamphuis, E., et alv Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood, 2006. 108(10): p. 3253-61.
Wang, X., et al.; SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol, 2020.
Tan, L., et al., Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther, 2020. 5: p. 33.
Chen, L.N., et alv Dysregulated renin-angiotensin system contributes to acute lung injury caused by hind-limb ischemia-reperfusion in mice. Shock, 2013. 40(5): p. 420-9.
Schalekamp, M.A. and A.H. Danser, How does the angiotensin II type 1 receptor 'trump1 the type 2 receptor in blood pressure control? J Hypertens, 2013. 31(4): p. 705-12.
Yu, Q.H., et al., Captopril pretreatment protects the lung against severe acute pancreatitis induced injury via inhibiting angiotensin II production and suppressing Rho/ROCK pathway. Kaohsiung J Med Sci, 2016. 32(9): p. 439-45.
Maron, B.A. and J.A. Leopold, The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 Grover Conference series). Pulm Circ, 2014. 4(2): p. 200-10.
Shroff, R., M. Wan, and L. Rees, Can vitamin D slow down the progression of chronic kidney disease? Pediatr Nephrol, 2012. 27(12): p. 2167-73.
Imai, Yv et al., Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature, 2005. 436(7047): p. 112-6.
Pinto, B.G., et al., ACE2 Expression is Increased in the Lungs of Patients with Comorbidities Associated with Severe COVID-19. medRxiv, 2020: p. 2020.03.21.20040261.
Hussain, A., B. Bhowmik, and N.C. do Vale Moreira, COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract, 2020. 162: p. 108142.
Kuba, K., et a I., A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med, 2005. 11(8): p. 875-9.
Kong, J., et al., VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol, 2013. 27(12): p. 2116-25.
