Cognition and vitamin D – summary of expert opinions
Vitamin D and Cognition in Older Adults': updated international recommendations
Accepted Date : 23-May-2014 Article type : Review
Cedric Annweiler 1'2, Erding Dursun3, Francois Feron4, Duygu Gezen-Ak3, Allan V. Kaluejf, Thomas Littlejohns6, David J. Llewellyn6, Pascal Millet4, Tammy Scott7, Katherine L. Tucker8, 3 1, Selma Yilmazer & Olivier Beauchet
From the department of Neuroscience, Division of Geriatric Medicine and Memory Clinic, UPRES EA 4638, UNAM, Angers University Hospital, Angers, France; Robarts Research Institute, Department of Medical Biophysics, Schulich School of Medicine and Dentistry, the University of Western Ontario, London, ON, Canada; Department of Medical Biology, Cerrahpasa Faculty of Medicine, Istanbul University, Istanbul, Turkey; 4Aix Marseille Universite, CNRS, NICN UMR 7259, 13916, Marseille, France; 5ZENEREI Institute, Slidell, LA, USA; 6University of Exeter Medical School, Exeter, UK; 7Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA; and 8Department of Clinical Laboratory and Nutritional Sciences, University of Massachusetts, Lowell, MA, USA
Correspondence: Cedric Annweiler, MD, PhD, Department of Neuroscience, Division of Geriatric Medicine, Angers University Hospital, 49933 Angers, France; e-mail: [email protected]; Phone: ++33241355486; Fax: ++33241354894
Table 1 Position of experts on key questions related to 'Vitamin D and cognition in older adults' (July 2013)
ADRD s = Alzheimer's disease and related disorders
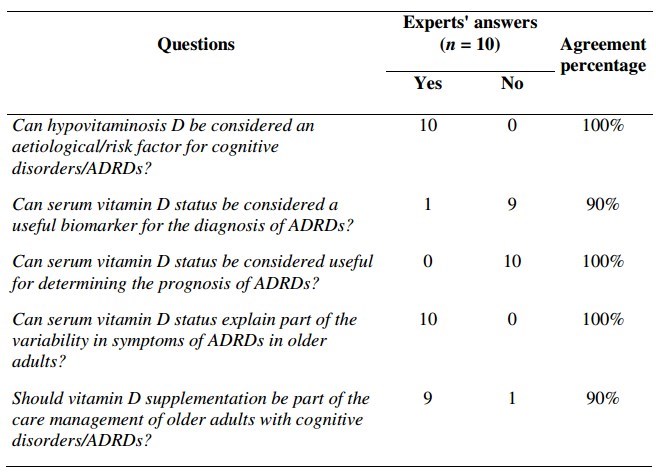
This table is at the end of this web page
Background. Hypovitaminosis D, a condition that is highly prevalent in older adults aged 65 years and above, is associated with brain changes and dementia. Given the rapidly accumulating and complex contribution of literature in the field of vitamin D and cognition, clear guidance is needed for researchers and clinicians.
Methods. International experts met at an invitational summit on 'Vitamin D and Cognition in Older Adults'. Based on previous reports and expert opinion, the task force focused on key questions relating to the role of vitamin D in Alzheimer's disease and related disorders. Each question was discussed and voted using a Delphi-like approach.
Results. The experts reached agreement that hypovitaminosis D increases the risk of cognitive decline and dementia in older adults and may alter the clinical presentation as a consequence of related comorbidities; however, at present, vitamin D level should not be used as a diagnostic or prognostic biomarker of Alzheimer's disease due to lack of specificity and insufficient evidence. This population should be screened for hypovitaminosis D because of its high prevalence, and should receive supplementation, if necessary; but this advice was not specific to cognition. During the debate, the possibility of 'critical periods' during which vitamin D may have its greatest impact on the brain was addressed; whether hypovitaminosis D influences cognition actively through deleterious effects and/or passively by loss of neuroprotection was also considered.
Conclusions. The international task force agreed on five overarching principles related to vitamin D and cognition in older adults. Several areas of uncertainty remain, and it will be necessary to revise the proposed recommendations as new findings become available.
Introduction
About 1 billion individuals worldwide are estimated to have hypovitaminosis D (i.e. inadequate levels of serum 25-hydroxyvitamin D (25OHD) below 75 nmol/L or 30 ng/mL) [1, 2]. In addition to regulating bone metabolism, vitamin D exerts multiple biological actions mediated by the nuclear hormone vitamin D receptor (VDR) [2-7]. New target organs, such as the central nervous system (CNS), and evidence for a neurosteroid action of vitamin D have been reported [3-8]. The need to explore the adverse impact of vitamin D deprivation on the ageing brain seems especially important because nearly one in two older adults (i.e., aged 65 years and above) [2] and 70-90% of adults with cognitive difficulties [9] have hypovitaminosis D. Together, this raises the possibility that vitamin D plays a role in the natural history of cognitive dysfunction, including Alzheimer's disease (AD) and related disorders (ADRDs) [9-13].
Because of the rapidly accumulating and complex contribution of both basic and clinical research in this field, an invitational summit of leading experts was convened in order to (i) identify areas clearly supported by the literature, (ii) nuance findings that remain not proven, (iii) raise new research questions, and (iv) provide clear guidelines to the medical and scientific communities.
Methods
Consensus finding at the invitational summit of experts consisted of a three-step process. In the first step, in February 2013, the first author (CA) invited leading international experts, including physicians and scientists from a wide range of disciplines identified on the basis of their relevant publications and citations, to form an international task force. All invited experts accepted the need for a task force and agreed to meet at an invitational summit.
In the second step, all experts communicated by email with the first author to better identify respective areas of expertise, discuss unmet needs and potential learning objectives in the field, and propose a holistic approach to the issue of 'vitamin D and cognition' based on the expertise of all participants. After discussion, the following programme was approved by all: (i) neurosteroid properties of vitamin D (KLT and TS); (ii) contribution of transgenic animal models to understanding the neurological effects of vitamin D (AVK); (iii) vitamin D and ADRDs: lessons from animal models (FF); (iv) brain changes associated with hypovitaminosis D (CA); (v) polymorphisms of vitamin D receptors: clinical implication for human variants (ED and DGA); (vi) cognitive disorders and hypovitaminosis D: observational approach (DJL and TL); and (vii) vitamin D intake and cognition: interventional approach (OB).
In the third step, the task force met in person on 15 July 2013 at the invitational summit in Boston, MA, USA. After presentations relating to each aspect of the programme, group discussions were held to attempt to identify consensus and disagreements, in order to highlight knowledge gaps and to emphasize key messages for the scientific and medical communities. Finally the discussion focused on five key questions regarding the implications of vitamin D as a risk factor for ADRDs, as a diagnostic and/or prognostic biomarker of ADRDs, as an explanation of the variability of the clinical manifestations of ADRDs, and the potential benefits of vitamin supplementation D in the management of ADRDs (Table 1). The 10 experts voted on each key questions: 'yes' (agreement) or 'no' (disagreement). It was agreed that questions supported by >75% of 'yes' votes would be immediately accepted whereas those with <25% would be rejected outright. Others would be subjected to further discussion and subsequent voting, where >67% support or, in an eventual third round, a majority of >50% would be needed for acceptance. After the face-to-face meeting, the statements were distributed to the committee members by email for final comments. Only suggestions for improvements for clarity of wording or to address redundancies were considered, while changes to the meaning were not accepted.
Results
Overview of the research topic
At the beginning of the summit, the experts discussed the current status of the research topic.
Neurosteroid properties of vitamin D: involvement in neurophysiology
Vitamin D enters the cerebrospinal fluid (CSF) and brain by crossing the blood-brain barrier (BBB) via passive diffusion and via specific carriers in the cerebral capillaries or the blood-CSF barrier in the plexus choroideus [14, 15]. The concentration of 25OHD (circulating form of vitamin D) in the CSF positively correlates with that in the serum under physiological conditions [14]. In situ, vitamin D exerts most of its actions through its nuclear hormone receptor the VDR, which is expressed in neuronal and glial cells in almost all regions of the CNS. In particular, the VDR is expressed in the hippocampus, hypothalamus, cortex and subcortex [5-7], the areas essential for cognition. The active form of vitamin D, 1,25-dihydroxyvitamin D (1,25OHD), has a trophic function of neuronal differentiation and maturation via control of the synthesis of neurotrophic agents such as nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF) [7, 16]. It also accelerates neuronal growth in rat hippocampal cell cultures [16]. Moreover, 1,25OHD regulates the genetic expression of numerous neurotransmitters in the brain, including acetylcholine, dopamine, serotonin and y-aminobutyric acid, notably in the hippocampus [4, 5].
Neurosteroid properties of vitamin D: neuroprotective action.
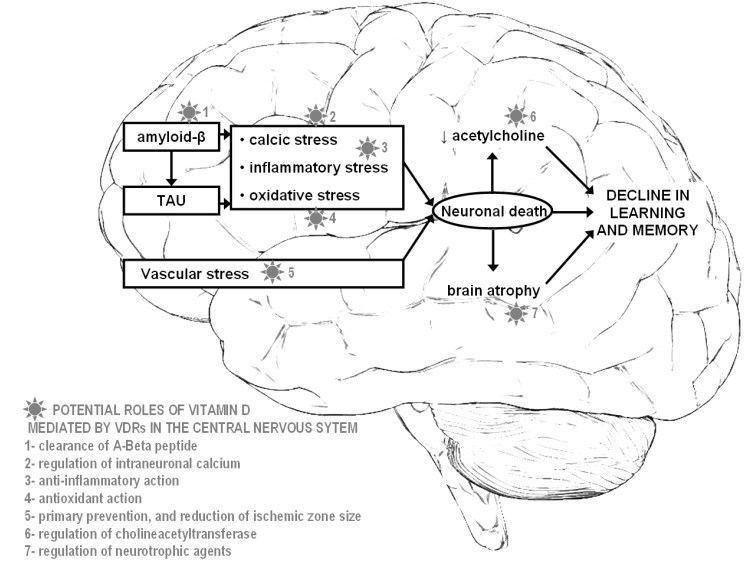
Vitamin D appears to enhance neuronal defense. Studies in mice have demonstrated that vitamin D may reverse age-related inflammatory changes in the hippocampus [17]. Vitamin D has also been shown to attenuate amyloid-fi (Afi) 42 accumulation by stimulating phagocytosis of the Afi peptide [ 18], and enhance brain-to-blood Afi efflux transport at the BBB [ 19], resulting in a decreased number of amyloid plaques [20]. In addition, it was reported that vitamin D regulates intra-neuronal calcium homeostasis via the regulation of voltage-gated calcium channels [21], including those targeted by Afi [22], supporting the idea that vitamin D has the potential to rearrange neuronal calcium homeostasis altered by the Afi peptide. Finally, vitamin D was shown to exhibit neuroprotective properties against glutamate toxicity [23, 24] through the upregulation of VDR expression [23] and antioxidant effects [24]. Indeed, it has been reported that vitamin D protects against free radicals generated by reactive species of oxygen [24] and nitric oxide [25], inhibits the synthesis of inducible nitric oxide synthase [26] and regulates the activity of the y-glutamyl transpeptidase [4], which is a key enzyme involved in the antioxidant metabolism of glutathione.
Interestingly, these effects relate to mechanisms implicated in the pathogenesis of AD (Fig. 1), which suggests that the lack of vitamin D may explain at least part of the natural history of this disease [27].
Hypovitaminosis D and/or inefficient utilization of vitamin D: which is most important?
Accumulating evidence suggests that hypovitaminosis D could be associated with the pathogenesis of AD. However, based on recent findings from in vitro studies that the vitamin D pathway is the target of Ai-induced toxicity in AD, this question can be addressed in a different way. Essentially, Ai renders neurons deficient in vitamin D by increasing the degradation of active vitamin D and VDR [22, 28]. This may be referred to as 'inefficient utilization of vitamin D'. Ai may disrupt the utilization of vitamin D in the brain even if the systemic concentration meets the requirements of the brain. Any alteration in genes related to the action of vitamin D, including its receptors (VDR and 1,25-MARRS [membrane associated, rapid response steroid-binding]), enzymes related to its metabolism or transporters, may also result in inefficient utilization of vitamin D, thus making neurons vulnerable to neurodegeneration [22, 28-32]. This possibility is supported by the association between AD and polymorphisms of VDR and megalin [30, 31, 33] and the toxic effects of VDR and 1,25-MARRS suppression in neurons [29, 32, 34]. Although hypovitaminosis D and inefficient utilization of vitamin D seem to be two different variables, crosstalk between the two should not be ignored when considering the pathogenesis of AD.
Consequences of the inefficient utilization of vitamin D on phenotype: the VDR knockout mouse model
The VDR knockout (VDR-/-) mouse is a powerful model of disrupted vitamin D-VDR signalling and inefficient utilization of vitamin D in the CNS. Of note, several robust phenotypes of the VDR-/- mouse have been revealed. In particular, accelerated ageing was clearly observed in VDR-/- mice [35], which may contribute to cognitive and behavioural deficits in these mice. Consistently, mild cognitive deficits have been shown in VDR-/- mice [36]. Elevated anxiety and neophobia were also seen across multiple tests in these mice on several different background strains [37]. Altered instinctive and social behaviours, evidenced by aberrant maternal behaviours, were also reported [38]. In addition, progressive decline in sensory abilities (especially hearing and balancing) of VDR-/- mice [39] together with several motor abnormalities [37, 40] have been demonstrated. These factors may also contribute non-specifically to overall cognitive decline and aberrant phenotypes.
In general, findings from mouse models seem to parallel accumulating clinical evidence in various neurobehavioural domains. The specific issue of changes in the brain (i.e. the organ that supports high-level functions) related to vitamin D deprivation should be considered.
Consequences of vitamin D deprivation on brain structures
Few studies have investigated the effect of inadequate vitamin D status on brain structures. In terms of brain volumetry, it was shown that rats born to vitamin D-deficient mothers had profound alterations in the brain at birth compared to control animals, with a thinner cortex and enlargement of the lateral ventricles [41]. In humans, no significant difference in whole-brain volume and sulcal grade according to serum vitamin D levels was observed in one study [42], whereas in another one an association between vitamin D concentration and ventricular volume (a proxy for brain atrophy) was found in older adults [43]. This difference only applied to the main ventricular bodies, and not to the temporal horns. Consistently, no difference in hippocampal volume was reported according to vitamin D levels among older community dwellers in the former study [42].
Studies in rats to examine brain vascular changes showed that vitamin D attenuated cortical infarction induced by cerebral arterial ligation [44]. In humans, a meta-analysis showed clearly, in both cross-sectional and longitudinal studies, that the risk of stroke was increased with hypovitaminosis D [45]. Of interest, this finding was also true for more subtle changes such as white matter damage [42], which is thought to disrupt cortical-subcortical white matter tracts that connect important cognitive regions of the brain [46]. Thus, it is possible that cerebrovascular changes linked to hypovitaminosis D could explain some higher-level disorders in older adults.
Vitamin D deprivation and cognitive disorders: observational approach in older adults
Recent large and representative epidemiological population-based studies have been conducted to examine the relationship between hypovitaminosis D and cognitive disorders/ADRDs [9]. Subsequent meta-analyses confirmed that a low vitamin D concentration in older adults is associated with reduced cognitive performance [10, 12, 13], and is more prevalent in those with AD [11, 12]. Nevertheless, the possibility of reverse causality remains a major concern; in other words, does hypovitaminosis D contribute to cognitive decline or does cognitive decline lead to hypovitaminosis D? [27]. Importantly, further longitudinal prospective studies [47-49] have helped to establish the temporal sequence between hypovitaminosis D and cognitive disorders, showing that older individuals with lower vitamin D concentrations had a significantly increased risk of global cognitive decline and executive dysfunction compared to those with higher concentrations [13]. Moreover, in addition to effects on cognitive decline, preliminary studies confirmed that low vitamin D was associated with an increased risk of AD [50] and incident all-cause dementia [51]. Overall, there is evidence from observational studies indicating that low vitamin D concentrations contribute to the occurrence of cognitive decline and dementia in older adults.
Vitamin D supplements and cognition: interventional approach
Few intervention studies have been conducted specifically to test the effect of vitamin D supplements on cognitive function. Of interest, observational studies have reported that a higher intake of vitamin D (whether from food, supplements or sun exposure) is associated with better cognitive function in older individuals [27]. For instance, consuming more than\ 800IU of vitamin D per day resulted in a 5-fold reduction in the risk of AD after 7 years of follow-up [52]. This neuroprotective effect has been further confirmed by before-after studies and quasi-experimental studies reporting cognitive improvement after vitamin D supplementation in the general population [53] as well as in patients who already have symptoms of ADRDs [54]. The cognitive benefits of supplementation appear from 4 weeks [55], and seem to be particularly strong for executive function and processing speed [13]. Supraphysiological doses may not be necessary to exert a cognitive effect [54], and consensual supplementation, with the objective to raise the concentration of 25OHD above 30 ng/mL (i.e. 75 nmol/L), appears to be sufficient [56].
Consensus finding
Following this overview of the different aspects of 'vitamin D and cognition in older adults', the experts expressed and discussed their position on the following five key questions (Table 1).
Can hypovitaminosis D be considered an aetiological/risk factor for cognitive disorders/ADRDs?
In response to the first question (Table 1), all experts agreed that hypovitaminosis D (and/or the inefficient utilization of vitamin D) can be considered a risk factor for cognitive decline and for dementia in general. This notion is supported in part by experimental evidence showing an action of vitamin D in the CNS [3-8], and primarily by prospective longitudinal studies in humans, which have consistently demonstrated that vitamin D concentration in older adults is associated with subsequent cognitive change and, in particular, that hypovitaminosis D predicts incident occurrence of cognitive decline and dementia [13, 4851]. In addition, it is noteworthy that several preclinical studies have shown that the genetic polymorphism of VDR explains the existence of responders and non-responders to vitamin D, and modulates the neuroprotective efficiency of vitamin D, thus explaining a higher rate of ADRDs in non-responders [30, 31]. For instance, a significant association was shown between the VDR gene APA1 polymorphism and the occurrence of AD, with the 'Aa' genotype increasing by 2.3-fold the risk of developing AD compared to the 'AA' genotype [30]. Also in the same study, the ' AATT' combined genotype was found to be less common in patients with AD than in healthy control subjects [30].
Can serum vitamin D status be considered a useful biomarker for the diagnosis of ADRDs?
In response to the second question (Table 1), all experts except one (CA) agreed that the concentration of serum 25OHD cannot be used as a biomarker of ADRDs. The arguments in favour of its use as a biomarker were that 25OHD is the most effective indicator of vitamin D status and can be considered as a 'biological indicator characterizing pathological processes' according to the definition of the World Health Organization [57]. For example, in a previous meta-analysis, it was shown that the probability that an individual without AD would have a higher serum 25OHD concentration than an individual with AD was 140% if both were selected randomly from a population [11]. The arguments against the use of serum 25OHD as a biomarker were that hypovitaminosis D is too common in older adults [1, 2] and that it is not sufficiently specific to be an efficient biomarker of ADRDs, or to be useful for the screening or diagnosis of ADRDs or for evaluating the response or tolerance to medical treatment. After discussion, the expert (CA) agreed that a systematic serum vitamin D determination is not justified as a biomarker for the diagnosis of ADRD, but added that the prevalence of hypovitaminosis D is sufficiently high in those with cognitive disorders/ADRDs that a vitamin D assay is justified in this population to detect and supplement the probable hypovitaminosis D. Finally, the group noted that the potential of other vitamin D-related proteins such as the vitamin D-binding protein (VDBP) should not be dismissed, and evaluation in larger studies is required [58, 59].
Can serum vitamin D status be considered useful for determining the prognosis of ADRDs?
In response to the third question (Table 1), all experts agreed that it is impossible at present to determine whether vitamin D status is a prognostic marker for ADRDs, as no studies have yet examined the risk of cognitive decline according to vitamin D status in patients with ADRDs. Specifically, it is unclear whether patients with hypovitaminosis D progress more quickly to a more severe stage of dementia than patients with higher vitamin D levels. Of note, such studies in non-demented individuals have shown that hypovitaminosis D predicted a faster and greater cognitive decline compared to higher vitamin D levels [47-49], suggesting that this may also be the case in patients with ADRDs. It was stated that studies addressing this specific issue are required.
Can serum vitamin D status explain part of the variability in symptoms of ADRDs in older adults?
In response to the fourth question (Table 1), all experts agreed that vitamin D can explain, at least in part, the diversity of symptoms in ADRDs. First, hypovitaminosis D affects many organs other than the brain, and has been associated with numerous diseases such as hypertension, type 2 diabetes, vascular disease and osteoporosis, as well as the propensity to fall [1, 2] (Fig. 2). All these conditions may be found in patients with both ADRDs and hypovitaminosis D, thus affecting patients' functional independence and the clinical presentation. Secondly, on the basis of animal experiments using VDR-/- mice, it appears that genetic polymorphisms of VDR may regulate the efficiency of vitamin D use, resulting not only in cognitive but also in various non-cognitive manifestations [35-40]. It was thus concluded that alterations in the vitamin D/VDR axis might explain part of the variability in clinical characteristics observed in ADRDs.
Should vitamin D supplementation be part of the care management of older adults with cognitive disorders/ADRDs?
In response to the final question (Table 1), all experts except one (TS) agreed that vitamin D supplements should be part of the care management of older adults with cognitive disorders/ADRDs (Table 1). The arguments against vitamin D supplementation in this population were based on the small number of clinical trials in the field, and the lack of well-conducted randomized clinical trials (RCTs) to test the effectiveness of vitamin D supplements against placebo in patients with ADRDs [27]. Arguments in favour of this option were discussed. First, the results of the few reported clinical trials were generally positive [53-56]. In particular, despite their design limitations, before-after studies have shown cognitive improvements, mainly of executive functions, after vitamin D supplementation, as highlighted by a meta-analysis [13]. Secondly, while vitamin D repletion has positive effects on the brain as well as on a number of other organs [1, 2], with substantial benefits in terms of survival [60, 61], no adverse effects have been reported for consensual supplementation [62], which reduces the risk of this practice. After discussion, the expert (TS) agreed that patients with both cognitive decline/ADRDs and hypovitaminosis D should receive vitamin D supplementation, but added that this advice is not specific for cognitive decline/ADRDs and is justified by all the expected bone and non-bone effects of vitamin D supplementation. This addition was approved by all experts.
Discussion
The summit enabled international experts to adopt a common position on five issues of primary importance for clinicians and researchers interested in the relationship between vitamin D and cognition. It was concluded that hypovitaminosis D and the inefficient utilization of vitamin D increase the risk of cognitive decline/ADRDs in older adults and may alter the clinical presentation of the disease, particularly as a consequence of accompanying morbidities; however current evidence is insufficient to recommend hypovitaminosis D as a reliable diagnostic or prognostic biomarker of cognitive decline/ADRDs. Moreover, the experts recommended correcting hypovitaminosis D in individuals with cognitive decline/ADRDs. However, this advice was not specific for cognitive decline/ADRDs, and further well-conducted RCTs are required to test more specifically the causal relationship between hypovitaminosis D and cognitive decline/ADRDs. Finally, the following new approaches and research questions were debated.
The need to consider both brain and non-brain effects of vitamin D when examining cognition
Ageing is accompanied by an increased incidence of both dementia and chronic conditions, such as cardiovascular disease, neurosensorial deficits and osteoporosis [63]. In fact, there is a close inter-relationship between these disorders. Dementia may alter the prognosis and management of other chronic diseases. On the other hand, chronic diseases can precipitate the evolution of dementia, changing the clinical presentation and accelerating the loss of independence, which is the major determinant of patients' quality of life. Of note, the action of vitamin D is not confined to the brain; vitamin D also affects multiple other tissues and organs, and age-related hypovitaminosis D has been associated with a number of conditions, including vascular disease, hearing loss, loss of visual acuity and osteoporosis [1, 2, 64]. This complicates the interpretation of the impact of vitamin D on cognition. The different possible interactions between sociodemographic characteristics of older adults, their vitamin D status and the brain and non-brain determinants of dementia are shown in Fig. 2. To better understand the effect of vitamin D on these interactions, a model was proposed (Fig. 3) in which A, the cognitive performance, is 'balanced' against B, the performance/health/function of another organ/tissue of the body that is related to neurocognition. Scenario 0 in Fig. 3 represents the ground state, with basal functioning of cognition (A) and of the other condition related to neurocognition (B). Four other scenarios are shown in which the A/B balance changes following vitamin D supplementation. The first scenario corresponds to an overall improvement in both A and B (e.g. improvement in hypertension and cognition following addition of vitamin D) [65]. The second scenario corresponds to an overall deterioration in A and B (e.g. vascular calcification and deterioration in cerebrovascular health following addition of vitamin D) [66]. The third scenario corresponds to an improvement in A but a deterioration in B (e.g. cognitive enhancement but transient increased risk of falls following addition of a high dose of vitamin D) [67]. Finally, the fourth scenario corresponds to an improvement in B accompanied by a deterioration in A (e.g. increased calcium absorption in the digestive tract following addition of vitamin D with a consequent ameliorative effect on secondary osteoporosis, but a deleterious effect on vascular calcification and cerebrovascular health) [66]. Thus, due to the ubiquitous role of vitamin D, elucidating its cognitive effects is more complex than investigating only the cerebral actions, and future basic and clinical studies should take into account all the effects of vitamin D in the body, particularly with regard to morbidity burden.
The 'critical periods' hypothesis
The discussion of the action of vitamin D in the CNS also raised the possibility of 'critical periods of life' during which vitamin D may have the greatest impact on the CNS and during which sufficient vitamin D would be essential. Previous studies have shown that variables related to the development of ADRDs are present at particular times of life, commonly long before the onset of disease [68]. A proposed representation of neurocognitive function is shown graphically in Fig. 4A. We have identified a period of rapid neurobehavioural development during the prenatal stage (especially gain of brain volume, and also gain of brain function) and younger ages (especially gain of function, and also gain of volume), a plateau phase corresponding to adulthood, and finally a phase of brain volume and function loss corresponding to older ages. In this model, the curve is similar to that of the well-known lifetime changes in bone mass [69]. For prevention of osteoporosis in women, it is important to (i) reach a peak in bone mass as high as possible at the end of the growth period, and (ii) reduce the slope of decline as much as possible after the menopause [69]. With similar logic, based on the neurophysiological effects of vitamin D, we suggest that there are three critical periods in which cognitive impairment in the elderly can be prevented: (i) during the prenatal period when vitamin D may have a beneficial effect on neurological development (Fig. 4B); (ii) at younger ages during which vitamin D would increase cognitive abilities and enhance cognitive reserve (Fig. 4C); and (iii) at older ages during which vitamin D would prevent neurocognitive loss (Fig. 4D).
These assumptions are consistent with previous experiments in animals. Specifically, it has been shown that rats born to vitamin D-deficient mothers had profound brain alterations at birth [41], consistent with altered signals for neuronal differentiation [41]. The continuing brain changes after restoration of a normal vitamin D-containing diet [70], together with the observation of abnormal adult behaviours [71], suggested that prenatal hypovitaminosis D disrupted not only brain development but also adult brain functioning. Additionally, in humans, although an association between cognitive disorders and hypovitaminosis D has been reported during the fetal period [72, 73] and older ages [9-13], no studies conducted among younger adults have found an association between vitamin D and cognitive performance [74]. This suggests that vitamin D may play a crucial role in enhancing neurocognition during fetal development, growth and senescence, but less of (or no) role during adulthood. Such suggestions should be confirmed by further studies, but could justify public health measures to systematically provide supplements for pregnant women, young children and elderly in order to prevent cognitive and behavioural disorders.
Vitamin D: is there an 'active' or 'passive' influence on neurocognition?
The attempt to elucidate the mechanism of action of vitamin D on neurocognition raised the issue of whether lower levels/inefficient utilization of vitamin D are causal factors that 'actively' trigger ADRDs or risk factors that 'passively' abolish CNS protection against ADRDs. The rationale for active involvement of hypovitaminosis D in the genesis of ADRDs is based on the involvement of vitamin D in neurophysiology (i.e. its neurotrophic role and the regulation of neurotransmitters) [4-8], with the possibility that vitamin D insufficiency results in a pathological dysfunction of the brain leading to ADRDs. An epidemiological argument against such active involvement is based on the finding that 70-90% of older adults have hypovitaminosis D but most do not suffer from ADRDs [2]. Thus, the possibility should be considered that vitamin D is instead a neuroprotective agent, as suggested by its antioxidant and anti-inflammatory effects [4-8], and its insufficiency could 'passively' lead to increased CNS sensitivity with a reduced response to 'dementiogenic' stress. Although this question of whether the influence on neurocognition is 'active' or 'passive' may appear trivial, the difference is in fact crucial because it affects our future approach to vitamin D repletion. Indeed, just enough supplementation to correct hypovitaminosis D should be sufficient if vitamin D is considered to be a neuroprotectant, whereas much higher doses may be considered, with the aim of boosting mental faculties, if vitamin D 'actively' controls the CNS. Furthermore, determining whether hypovitaminosis D is actively or passively involved in the history of ADRDs will help to define the objectives and outcomes of future RCTs. In other words, should vitamin D supplementation be tested for preventing the occurrence of ADRDs, or for reducing its symptoms? And could hypovitaminosis D be involved in the resistance to standard anti-dementia treatments? If hypovitaminosis D explains in part the pathological process of ADRDs, it may also enhance the effectiveness of standard anti-dementia treatments or account at least partially for the resistance to these treatments. Although speculative, this suggests that clinicians should restore vitamin D levels before starting anti-dementia treatments or use vitamin D as an adjunct to standard treatments. In line with this, a recent 6-month controlled trial demonstrated that the combination of memantine+vitamin D was superior to either memantine or vitamin D alone in preventing cognitive decline among participants with AD [75]. In fact, those taking both treatments had a clinically relevant and statistically significant gain of 4 points on the Mini Mental State Examination score of cognitive function. These results were consistent with the finding of an in vitro study of less degeneration of cortical axons after exposure to Afi peptide or glutamate in microfluidic neuronal cultures enriched with memantine plus vitamin D compared to control medium and cultures enriched with either memantine or vitamin D alone [76].
CONCLUSIONS
In conclusion, this first task force on 'Vitamin D and Cognition in Older Adults' enabled international experts to reach agreement that hypovitaminosis D and the inefficient utilization of vitamin D increase the risk of cognitive decline/ADRDs in older adults and may alter the clinical presentation of the disease, particularly as a consequence of accompanying morbidities; however at present hypovitaminosis D should not be used as a diagnostic or a prognostic biomarker of cognitive decline/ADRDs due to lack of specificity and insufficient evidence. The experts also recommended measurement of serum 25OHD because of the high prevalence of hypovitaminosis D in this population and supplementation, if necessary. However this advice was not specific for ADRDs in the absence of well-conducted RCTs. Future studies should consider the effects of vitamin D on comorbidities, and explore the stimulating and/or protective effects of vitamin D on neurocognition at different stages of life. A stronger focus on the role of vitamin D-related genetic variance (e.g. in the genes encoding VDR, a-hydroxylase or VDBP) in humans will also be important. In parallel, conditional and tissue (e.g. brain)-specific VDR mutations are eagerly expected to assess their neurophenotypes. Critical evaluation (and bridging, if possible) the clinical and animal models using altered vitamin D systems available in the field (e.g. VDR-/-, developmental vitamin D deficit, acute and chronic vitamin D treatment or depletion) should also be carried out in order to best interpret neurocognitive and behavioural abnormalities associated with vitamin D deprivation. Focusing on the threshold concentration of 25OHD required to prevent these adverse events, and on the dose of supplementation required, will also be important.
Finally, it will be necessary to revise the current document in due course, probably within the next 3-5 years, when significant evidence is available regarding the different aspects of the recommendations. It is hoped that there will be an expansion of high-quality research activities that either corroborate or lead to modification of these recommendations.
REFERENCES are in attached PDF

Table 1 Position of experts on key questions related to 'Vitamin D and cognition in older adults'
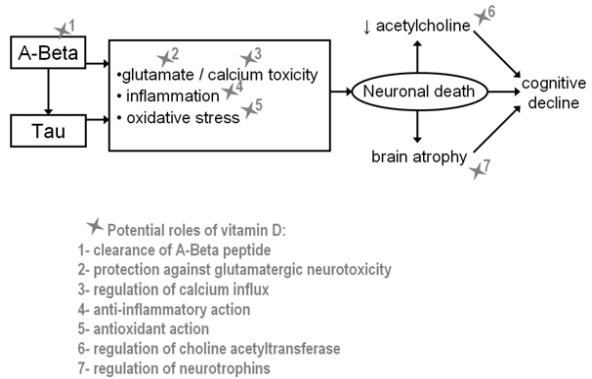
Fig. 1 Potential targets and neuroprotective roles of vitamin D during the natural history of Alzheimer's disease
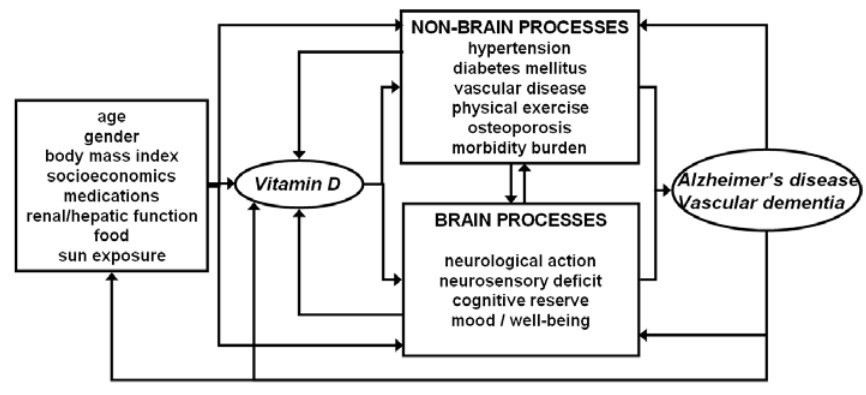
Fig. 2 Relationship between vitamin D and Alzheimer's disease/vascular dementia: an overview of the different conditions, mechanisms and interactions that may be involved. Arrows represent the causal direction of the influences.
Fig. 3 Schematic diagram of the possible influences of vitamin D supplements on the balance between cognition (A) and comorbidities (B). See text for further details and examples.
Fig. 4 Schematic diagram of the evolution of neurocognitive health throughout life (A), and the possible influence of vitamin D at potential critical periods: prenatal (B), younger ages (C) and older ages (D).
See also Vitamin D Life
- Alzheimer’s disease – vitamin D looks promising – Annweiler Jan 2014 - (previous publication by the same author) has the following chart
Search Vitamin D Life for Annweiler 105 items as of July 2014
Overview Alzheimer's-Cognition and Vitamin D has the following summary
{include}
The TOP articles in Cognitive and Vitamin D are listed here:
{category}
