COVID in hospital stopped by Vitamin D Receptor activators (curcumin, quercetin) – RCT
NASAFYTOL® supplementation in adults hospitalized with COVID-19 infection: results from an exploratory open-label randomized controlled trial
Front. Nutr. 10:1137407. doi: 10.3389/fnut.2023.1137407
Jean Gérain1, Melanie Uebelhoer2*, Bérénice Costes2, Julie Herman2, Sandra Pietri2, Anne-Françoise Donneau3, Justine Monseur3 and Yves Henrotin2 Belgium
25 people got Curcumin (42 mg), Quercetin (65 mg), Vitamin D (90 IU) daily

Objectives: The effect and safety of Nasafytol®, a food supplement combining curcumin, quercetin, and Vitamin D, on hospitalized COVID-19-positive patients as support to standard of care were to be assessed.
Methods: This exploratory, open-label, randomized, controlled trial was carried out among hospitalized adults with COVID-19 infection. Participants were randomly assigned to receive Nasafytol® or Fultium® control. The improvement of the clinical condition and occurrence of (serious) adverse events were evaluated. The study was registered on clincaltrials.gov with the identifier NCT04844658.
Results: Twenty-five patients received Nasafytol®, and 24 received Fultium®. Demographic characteristics were well balanced between the groups. On day 14 (or at hospital leave if <14days), no difference was observed between groups regarding their clinical condition, fever, or the need of oxygen therapy.
At day 7, however,
19 participants had been discharged from the hospital in the Nasafytol® arm compared to
10 participants in the Fultium® arm.
No participants were transferred to the ICU or died in the Nasafytol® arm, vs.
4 transfers and 1 death in the Fultium® arm.
The clinical condition of participants in the Nasafytol® arm had improved, as evidenced by a decrease in the COVID-19 WHO score. Interestingly, five SAEs occurred with Fultium®, while no SAE was observed with Nasafytol®.
Conclusion: Supplementation with Nasafytol®, in addition to standard-of-care treatment, led to a faster discharge from the hospital, improved clinical conditions of participants, and a reduced risk of serious outcomes, including transfer to the intensive care unit or death, in patients hospitalized with COVID-19.
📄 Download the PDF from Vitamin D Life
Vitamin D Life - studies in both categories Virus and Vitamin D Receptor
Note: COVID protects itself by deactivating the VDR, thus stopping Vitamin D from getting to cells
This list is automatically updated
{category}
40+ Vitamin D Life VIRUS category studies have RCT in their title
Note: COVID proven to be stopped by Vitamin D, VDR, UVB (makes D), Omega-3, Probiotics, etc.
This list is automatically updated
{LIST()}
Vitamin D Life – COVID-19 treated by Vitamin D - studies, reports, videos
{include}
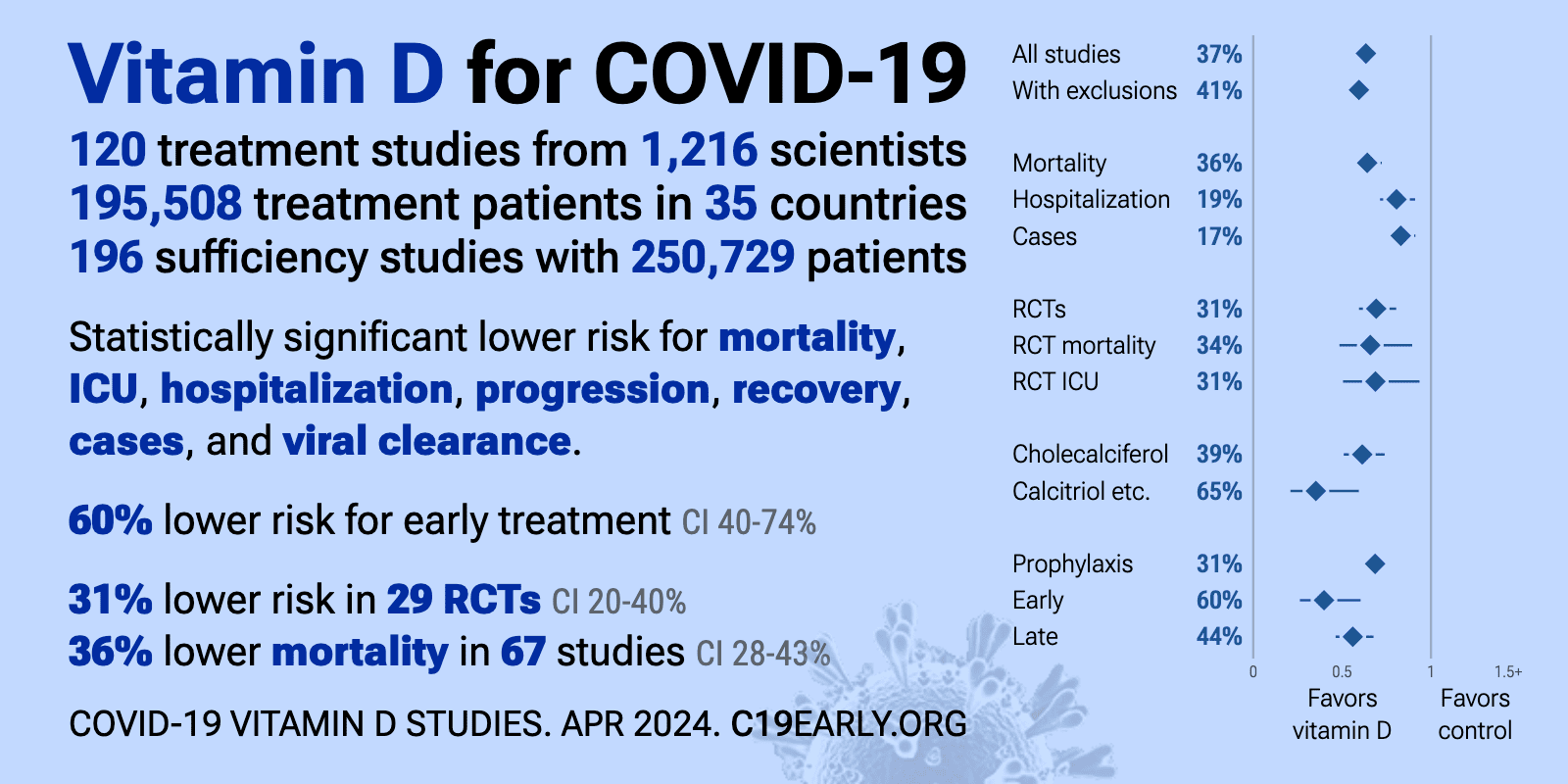
- The above image is automatically updated
Vitamin D Life – 26 health factors increase the risk of COVID-19 – all are proxies for low vitamin D includes
{include}
