18 fewer hospital days if given 500,000 IU of vitamin D while ventilated in ICU – RCT
High dose vitamin D administration in ventilated intensive care unit patients: A pilot double blind randomized control trial
Journal of Clinical & Translational Endocrinology, Available online 5 May 2016, doi:10.1016/j.jcte.2016.04.004
Jenny E. Han, MD, MSc1, 2, a, [email protected], Jennifer L. Jones, PhD, RD3, a, Vin Tangpricha, MD, PhD3, 6, Mona A. Brown, MSN, RN1, Lou Ann S. Brown, PhD4, Li Hao, MD3, Gautam Hebbar, MBBS, MPH3, Moon Jeong Lee3, Shuling Liu, PhD5, Thomas R. Ziegler, MD3, Greg S. Martin, MD,MSc1, 2
1 Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Department of Medicine, Emory University, Atlanta, GA
2 Emory Critical Care Center, Emory University, Atlanta, GA
3 Division of Endocrinology, Metabolism and Lipids, Department of Medicine, Emory University, Atlanta, GA
4 Department of Pediatrics, Division of Neonatal-Perinatal Medicine, Emory University, Atlanta, GA
5 Rollins School of Public Health, Emory University, Atlanta, GA
6 Atlanta VA Medical Center, Decatur, GA

Highlights
• First double blind RCT of vitamin D therapy in mechanically ventilated patients
• Treatment with placebo, 250000 IU or 500000 IU enteral vitamin D3 was well tolerated
• Significant increase in plasma 25(OH)D from baseline to day 7
• Significant decrease in hospital length of stay for vitamin D3 treated subjects
• No change in plasma LL-37 according to treatment group
Background: There is a high prevalence of vitamin D deficiency in the critically ill patient population. Several intensive care unit studies have demonstrated an association between vitamin D deficiency [25-hydroxyvitamin D (25(OH)D) < 20 ng/mL] and increased hospital length of stay (LOS), readmission rate, sepsis and mortality.
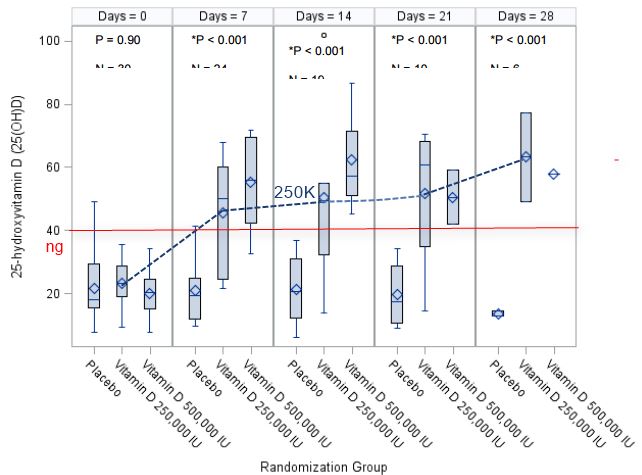
Material and Methods: Pilot, double blind randomized control trial conducted on mechanically ventilated adult ICU patients. Subjects were administered either placebo, 50,000 IU vitamin D3 or 100,000 IU vitamin D3 daily for 5 consecutive days enterally (total vitamin D3 dose = 250,000 IU or 500,000 IU, respectively). The primary outcome was plasma 25(OH)D concentration 7 days after oral administration of study drug. Secondary outcomes were plasma levels of the antimicrobial peptide cathelicidin (LL37), hospital LOS, SOFA score, duration of mechanical ventilation, hospital mortality, mortality at 12 weeks, and hospital acquired infection.
Results: A total of 31 subjects were enrolled with 13 (43%) being vitamin D deficient at entry (25(OH)D levels < 20 ng/mL). The 250,000 IU and 500,000 IU vitamin D3 regimens each resulted in a significant increase in mean plasma 25(OH)D concentrations from baseline to day 7; values rose to 45.7±19.6 ng/mL and 55.2 ± 14.4 ng/mL, respectively, compared to essentially no change in the placebo group (21±11.2 ng/mL), p<0.001. There was a significant decrease in hospital length of stay over time in the 250,000 IU and the 500,000 IU vitamin D3 group, compared to the placebo group (25 ± 14 and 18 ± 11 days compared to 36 ± 19 days, respectively; p=0.03). There was no statically significant change in plasma LL-37 concentrations or other clinical outcomes by group over time.
Conclusions: In this pilot study, high-dose vitamin D3 safely increased plasma 25(OH)D concentrations into the sufficient range and was associated with decreased hospital length of stay without altering other clinical outcomes.
www.clinicaltrials.gov (NCT01372995)
