100% not need COVID hospitalization with fluvoxamine plus one other drug – Thailand RCT
Early treatment with fluvoxamine, bromhexine, cyproheptadine, and niclosamide to prevent clinical deterioration in patients with symptomatic COVID-19: a randomized clinical trial
eClinicalMedicine Volume 70, April 2024, https://doi.org/10.1016/j.eclinm.2024.102517
Background
Repurposed drugs with host-directed antiviral and immunomodulatory properties have shown promise in the treatment of COVID-19, but few trials have studied combinations of these agents. The aim of this trial was to assess the effectiveness of affordable, widely available, repurposed drugs used in combination for treatment of COVID-19, which may be particularly relevant to low-resource countries.
Methods
We conducted an open-label, randomized, outpatient, controlled trial in Thailand from October 1, 2021, to June 21, 2022, to assess whether early treatment within 48-h of symptoms onset with combinations of fluvoxamine, bromhexine, cyproheptadine, and niclosamide, given to adults with confirmed mild SARS-CoV-2 infection, can prevent 28-day clinical deterioration compared to standard care. Participants were randomly assigned to receive treatment with fluvoxamine alone, fluvoxamine + bromhexine, fluvoxamine + cyproheptadine, niclosamide + bromhexine, or standard care. The primary outcome measured was clinical deterioration within 9, 14, or 28 days using a 6-point ordinal scale. This trial is registered with ClinicalTrials.gov (NCT05087381).
Findings
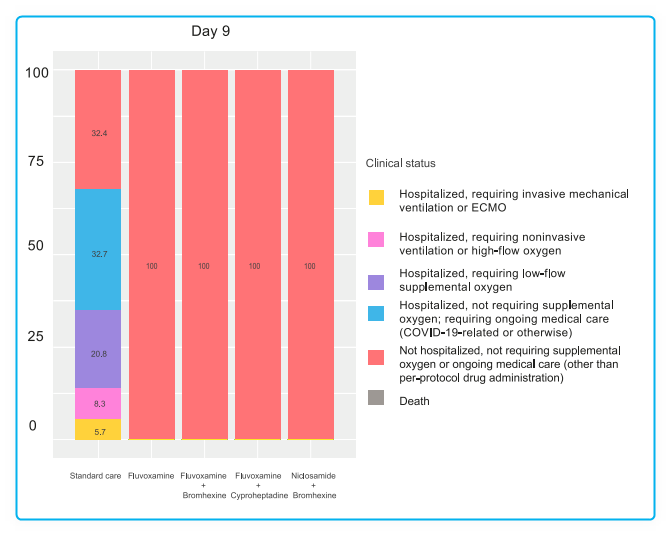
Among 1900 recruited, a total of 995 participants completed the trial. No participants had clinical deterioration by day 9, 14, or 28 days among those treated with
fluvoxamine plus bromhexine (0%),
fluvoxamine plus cyproheptadine (0%), or
niclosamide plus bromhexine (0%).
Nine participants (5.6%) in the fluvoxamine arm had clinical deterioration by day 28, requiring low-flow oxygen. In contrast, most standard care arm participants had clinical deterioration by 9, 14, and 28 days. By day 9, 32.7% (110) of patients in the standard care arm had been hospitalized without requiring supplemental oxygen but needing ongoing medical care. By day 28, this percentage increased to 37.5% (21). Additionally, 20.8% (70) of patients in the standard care arm required low-flow oxygen by day 9, and 12.5% (16) needed non-invasive or mechanical ventilation by day 28. All treated groups significantly differed from the standard care group by days 9, 14, and 28 (p < 0.0001). Also, by day 28, the three 2-drug treatments were significantly better than the fluvoxamine arm (p < 0.0001). No deaths occurred in any study group.
Compared to standard care, participants treated with the combination agents had significantly decreased viral loads as early as day 3 of treatment (p < 0.0001), decreased levels of serum cytokines interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β) as early as day 5 of treatment, and interleukin-8 (IL-8) by day 7 of treatment (p < 0.0001) and lower incidence of post-acute sequelae of COVID-19 (PASC) symptoms (p < 0.0001). 23 serious adverse events occurred in the standard care arm, while only 1 serious adverse event was reported in the fluvoxamine arm, and zero serious adverse events occurred in the other arms.
Interpretation
Early treatment with these combinations among outpatients diagnosed with COVID-19 was associated with lower likelihood of clinical deterioration, and with significant and rapid reduction in the viral load and serum cytokines, and with lower burden of PASC symptoms. When started very soon after symptom onset, these repurposed drugs have high potential to prevent clinical deterioration and death in vaccinated and unvaccinated COVID-19 patients.
📄 Download the PDF from Vitamin D Life
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) is approved by the (FDA) for the treatment of obsessive-compulsive disorder...
Vitamin D Life – COVID-19 treated by Vitamin D - studies, reports, videos
{include}
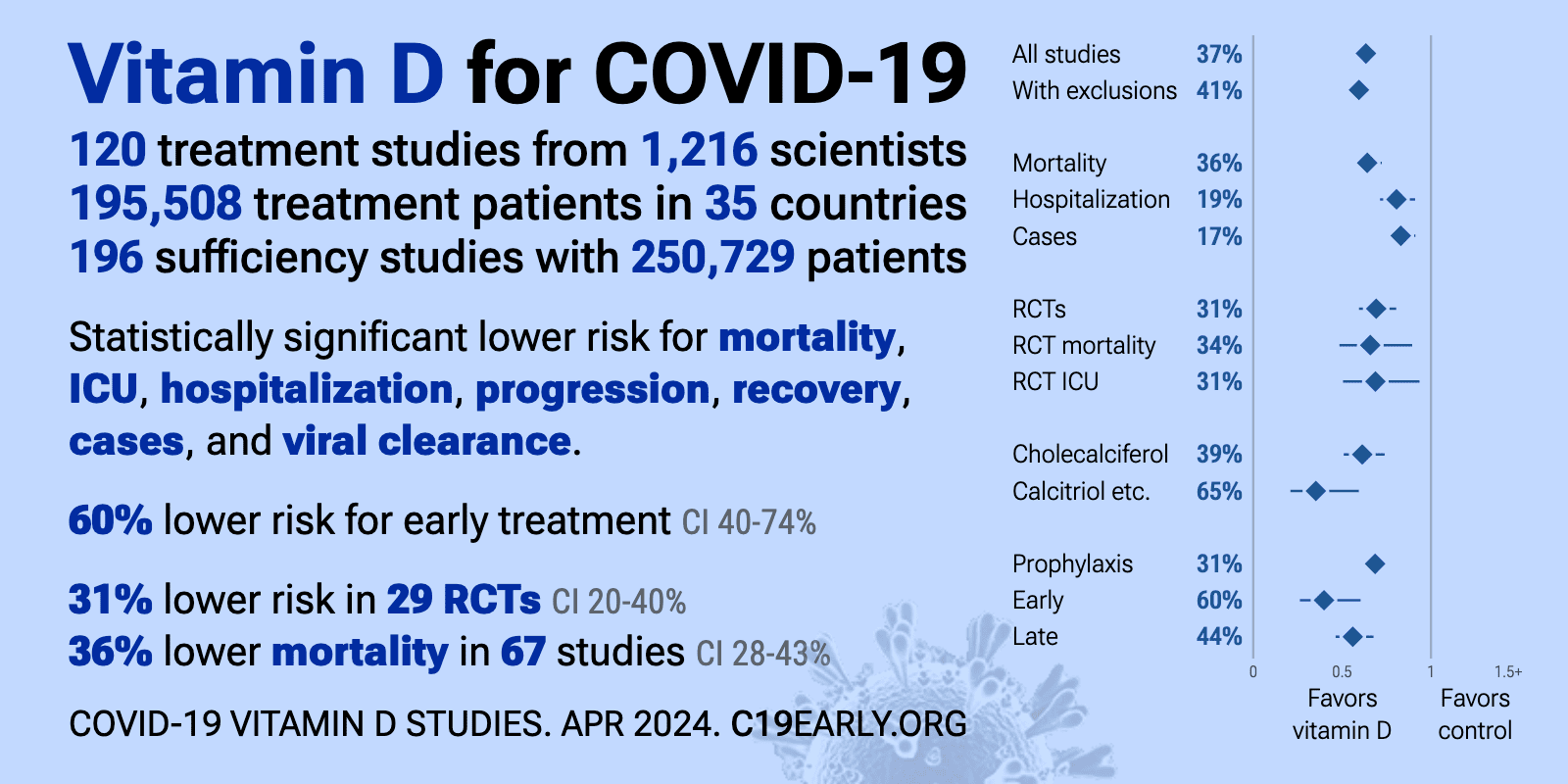
- The above image is automatically updated
4 treatment/prevention charts by C19early.org (Fluvoxamine similar to Vitamin D)
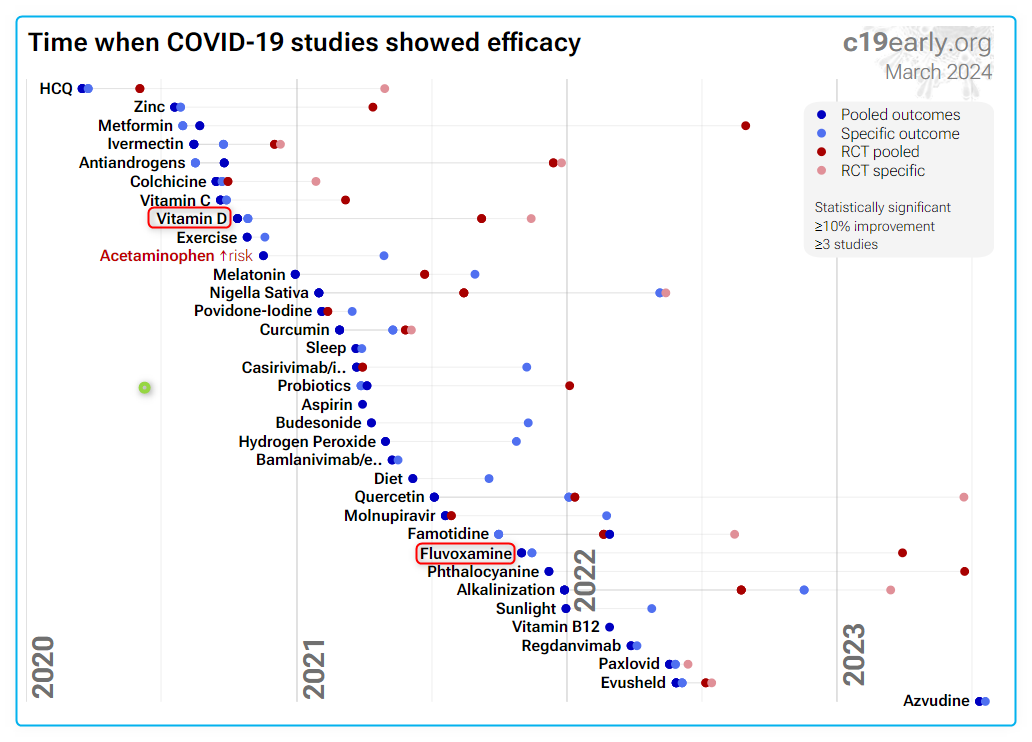

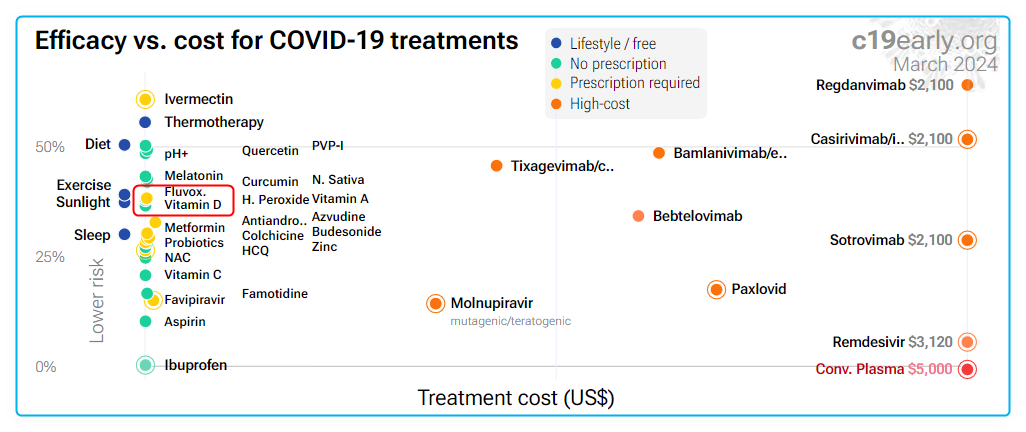
COVID prevention (methods with limited data have been deleted)

Fluvoxamine and COVID - Lancet RCT Jan 2022
Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial
Lancet DOI:https://doi.org/10.1016/S2214-109X(21)00448-4
- Gilmar Reis, PhD, Eduardo Augusto dos Santos Moreira-Silva, PhD, Daniela Carla Medeiros Silva, PhD, Prof Lehana Thabane, PhD, Aline Cruz Milagres, RN. Thiago Santiago Ferreira, MD, et al.
"Treatment with fluvoxamine (100 mg twice daily for 10 days) among high-risk outpatients with early diagnosed COVID-19 reduced the need for hospitalisation defined as retention in a COVID-19 emergency setting or transfer to a tertiary hospital."
📄 Download the PDF from Vitamin D Life
42+ Vitamin D Life Virus pages have RCT in the title
This list is automatically updated
{LIST()}
Very comprehensive description of this study by Robin on Substack
Note: FDA refused Emergency Use of fluvoxamine in early 2021: "benefits didn’t outweigh the risks"
Steve Kirsch Substack March 2024
