Vitamin D not help 10 days after COVID-19 symptoms - RCT
Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19. A Randomized Clinical Trial
JAMA. Published online February 17, 2021. doi:10.1001/jama.2020.26848
Igor H. Murai, PhD1; Alan L. Fernandes, PhD1; Lucas P. Sales, MSc1; et alAna J. Pinto, BSc2; Karla F. Goessler, PhD2; Camila S. C. Duran, MD1; Carla B. R. Silva, MD1; André S. Franco, MD1; Marina B. Macedo, MD, MSc1; Henrique H. H. Dalmolin, MD1; Janaina Baggio, MD1; Guilherme G. M. Balbi, MD1; Bruna Z. Reis, PhD1; Leila Antonangelo, MD, PhD3; Valeria F. Caparbo, PhD1; Bruno Gualano, PhD2,4; Rosa M. R. Pereira, MD, PhD1
Vitamin D not given until 10 days after symptoms (15 days after infection started?)
Key Points
Question What is the effect of a single high dose of vitamin D3 on hospital length of stay among hospitalized patients with moderate to severe coronavirus disease 2019 (COVID-19)?
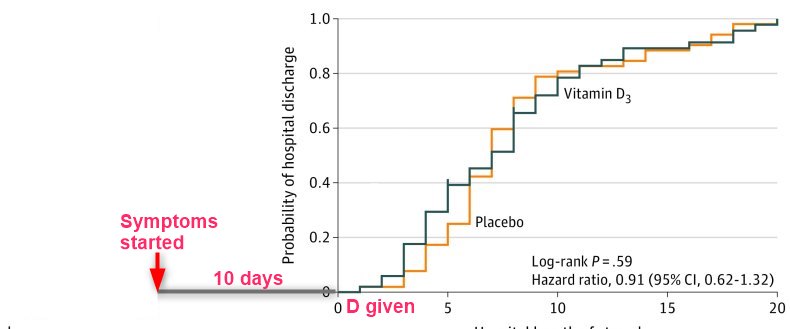
Findings In this randomized clinical trial that involved 240 hospitalized patients with moderate to severe COVID-19, a single dose of 200 000 IU of vitamin D3, compared with placebo, did not significantly reduce hospital length of stay (median of 7.0 vs 7.0 days; unadjusted hazard ratio for hospital discharge, 1.07).
Meaning The study does not support the use of a high dose of vitamin D3 for treatment of moderate to severe COVID-19 in hospitalized patients.
Importance The efficacy of vitamin D3 supplementation in coronavirus disease 2019 (COVID-19) remains unclear.
Objective To investigate the effect of a single high dose of vitamin D3 on hospital length of stay in patients with COVID-19.
Design, Setting, and Participants This was a multicenter, double-blind, randomized, placebo-controlled trial conducted in 2 sites in Sao Paulo, Brazil. The study included 240 hospitalized patients with COVID-19 who were moderately to severely ill at the time of enrollment from June 2, 2020, to August 27, 2020. The final follow-up was on October 7, 2020.
Interventions Patients were randomly assigned to receive a single oral dose of 200 000 IU of vitamin D3 (n = 120) or placebo (n = 120).
Main Outcomes and Measures The primary outcome was length of stay, defined as the time from the date of randomization to hospital discharge. Prespecified secondary outcomes included mortality during hospitalization; the number of patients admitted to the intensive care unit; the number of patients who required mechanical ventilation and the duration of mechanical ventilation; and serum levels of 25-hydroxyvitamin D, total calcium, creatinine, and C-reactive protein.
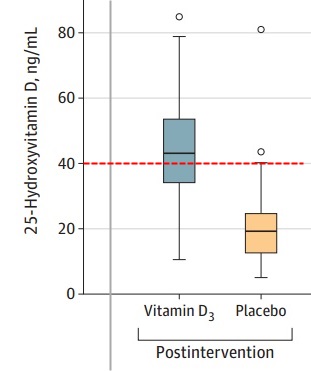
Results Of 240 randomized patients, 237 were included in the primary analysis (mean [SD] age, 56.2 [14.4] years; 104 [43.9%] women; mean [SD] baseline 25-hydroxyvitamin D level, 20.9 [9.2] ng/mL). Median (interquartile range) length of stay was not significantly different between the vitamin D3 (7.0 [4.0-10.0] days) and placebo groups (7.0 [5.0-13.0] days) (log-rank P = .59; unadjusted hazard ratio for hospital discharge, 1.07 [95% CI, 0.82-1.39]; P = .62). The difference between the vitamin D3 group and the placebo group was not significant for in-hospital mortality (7.6% vs 5.1%; difference, 2.5% [95% CI, –4.1% to 9.2%]; P = .43), admission to the intensive care unit (16.0% vs 21.2%; difference, –5.2% [95% CI, –15.1% to 4.7%]; P = .30), or need for mechanical ventilation (7.6% vs 14.4%; difference, –6.8% [95% CI, –15.1% to 1.2%]; P = .09). Mean serum levels of 25-hydroxyvitamin D significantly increased after a single dose of vitamin D3 vs placebo (44.4 ng/mL vs 19.8 ng/mL; difference, 24.1 ng/mL [95% CI, 19.5-28.7]; P < .001). There were no adverse events, but an episode of vomiting was associated with the intervention.
Conclusions and Relevance Among hospitalized patients with COVID-19, a single high dose of vitamin D3, compared with placebo, did not significantly reduce hospital length of stay. The findings do not support the use of a high dose of vitamin D3 for treatment of moderate to severe COVID-19.
Trial Registration ClinicalTrials.gov Identifier: NCT04449718
Preprint version of this is also on Vitamin D Life ((Severe COVID-19 not fought by vitamin D when given too late - RCT Nov 18, 2020)
📄 Download the PDF from Vitamin D Life
Editorial in the same issue noted some limitations of this study
" 208 participants they would have 80% power to detect a 50% difference in hospital length of stay, which is a highly improbable result"
"most of the patient population would be considered moderately ill and the results cannot be generalized to critically ill patients, who were excluded." Mechanical Ventilation and ICU
Comments on this trial
“ A recent study demonstrated that buckets of water made no difference in tackling a house fire, therefore we conclude there is no benefit to using water for firefighting ”."
a bucket of water can't put out a raging house fire... (but pretty useful for putting out the cigarette before it catches on the curtains though)
"There are considerable differences between the control and treatment groups in a number of variables known to be highly relevant to covid severity and hence length of stay. The treatment group had more people with diabetes who are more likely to die from covid."
The following would have been a useful vitamin D level if it had been achieved a week sooner
Note: The founder of Vitamin D Life enclosed 200,000 IU of Vitamin D in his Christmas cards
to be taken immediately if get COVID-19 symptoms Four capsules of 50,000 IU each on a small blister pack Amazon
COVID-19 treated by Vitamin D - studies, reports, videos
{include}
Virus meta-analyses
{category}
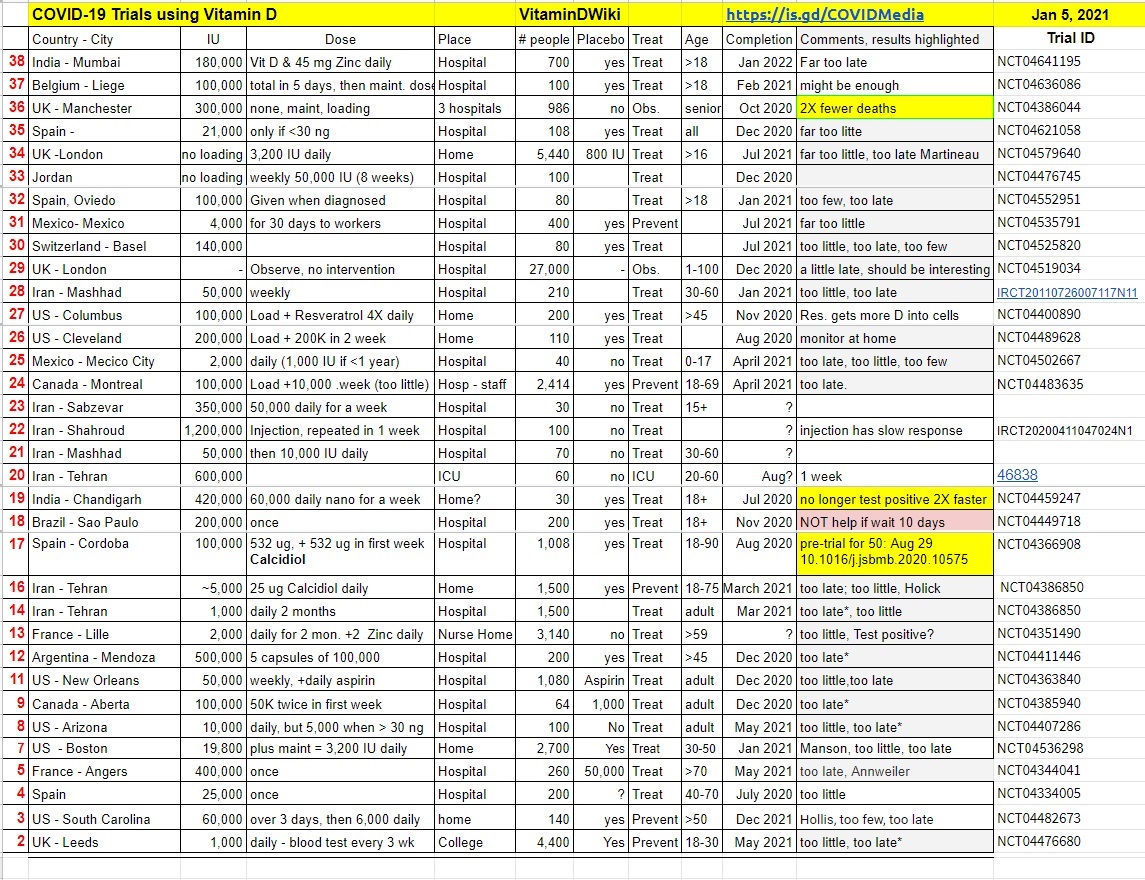
Many clinical trials are underway which are testing Vitamin D to fight COVID-19
Title change made Sept 2021 caused the visitor count to reset.
There have actually been visitors to this page since it was originally made
Short URL = https://is.gd/04449718
