Magnesium is vital to Vitamin D in 4 places (maybe 8)
Role of Magnesium in Vitamin D Activation and Function
J Am Osteopath Assoc. 2018;118(3):181-189 doi:10.7556/jaoa.2018.037
Anne Marie Uwitonze, BDT, MS; Mohammed S. Razzaque, MBBS, PhD
 --- 1. Magnesium and Vitamin D contains the following{include}
--- 1. Magnesium and Vitamin D contains the following{include}📄 Download the PDF from Vitamin D Life
Figure 4
Nutrients usually act in a coordinated manner in the body. Intestinal absorption and subsequent metabolism of a particular nutrient, to a certain extent, is dependent on the availability of other nutrients. Magnesium and vitamin D are 2 essential nutrients that are necessary for the physiologic functions of various organs. Magnesium assists in the activation of vitamin D, which helps regulate calcium and phosphate homeostasis to influence the growth and maintenance of bones. All of the enzymes that metabolize vitamin D seem to require magnesium, which acts as a cofactor in the enzymatic reactions in the liver and kidneys. Deficiency in either of these nutrients is reported to be associated with various disorders, such as skeletal deformities, cardiovascular diseases, and metabolic syndrome. It is therefore essential to ensure that the recommended amount of magnesium is consumed to obtain the optimal benefits of vitamin D.
The adequate balance of magnesium and vitamin D is essential for maintaining the physiologic functions of various organs. Vitamin D helps regulate calcium and phosphate balance to maintain healthy bone functions.1-6 Skeletal muscles, heart, teeth, bones, and many other organs require magnesium to sustain their physiologic functions. Furthermore, magnesium is needed to activate vitamin D. Abnormal levels in either of these nutrients can lead to serious organ dysfunctions.7-12 Magnesium is the fourth most abundant mineral in the human body after calcium, potassium, and sodium. Magnesium activates more than 600 enzymes and influences extracellular calcium levels.13 It is essential for the stability of cell function, RNA and DNA synthesis, and cell repair, as well as maintaining the antioxidant status of the cell. It is an important cofactor for the activation of a wide range of transporters and enzymes.14,15 Also, magnesium-dependent kinases are responsible for the activation of up to 30% of the functional body proteins. Approximately 40% of total body magnesium content is intracellular, and almost 60% of magnesium is present in bone and teeth, with less than 1% in extracellular fluids.15-20 Approximately 0.3% of total body magnesium is found in serum; therefore, serum magnesium concentration does not reflect the total amount of body magnesium content and is a poor predictor of intracellular magnesium content. 7,14,20-23- Even when the skeletal or intracellular magnesium content of soft tissue may be depleted, the circulating levels of magnesium could remain within the normal range because of its tight homeostatic control24; severely reduced tissue and bone magnesium content in the setting of normal serum magnesium levels has been termed chronic latent magnesium deficit.24
Vitamin D is a lipid-soluble vitamin with a steroidal structure that exerts numerous essential cellular and molecular functions. Other than bone mineralization, vitamin D is also involved in cellular differentiation and regeneration of various organs; it is claimed to influence glucose homeostasis and actively contribute to maintaining the physiologic functions of the musculoskeletal system. Adequate intake of vitamin D has shown to diminish the risk of some of the skeletal as well as nonskeletal disorders.25-32 Vitamin D needs to be converted from its storage or inactive form (25[OH] D) to an active form (1,25[OH]2D) before exerting its biological functions. These various stages of vitamin D conversions are actively dependent on the bioavailability of magnesium.33,34 Vitamin D is mostly synthesized from 7-dehydrocholesterol upon skin exposure to sunlight (>80%) and may also be obtained from dietary sources or supplements as either vitamin D2 or D3. Research has claimed that its dysregulation can lead to the development of numerous diseases, affecting the cardiovascular system, musculoskeletal system, and nervous system.35-39 Optimal health benefits of exogenous and endogenous vitamin D might not be achieved without the adequate presence of magnesium, as the bioactivity of vitamin D is a magnesium-dependent process.33,34 The purpose of this review article is to present the biological significance of magnesium in vitamin D metabolism and its therapeutic importance to minimize complications related to vitamin D deficiency.
Physiologic Regulation of Magnesium
Body storage of nutrients are partially dependent on the balance between daily intake and renal loss.
Approximately 30% to 70% of dietary magnesium is absorbed by the healthy intestine; the absorption rate increases with negative magnesium balance and with the high acidic microenvironment.
Magnesium homeostasis in the body is regulated by a delicate interplay among intestinal absorption, skeletal resorption, and renal reabsorption.7,40,41
Intestinal magnesium absorption is attained by a passive para- cellular and an active transcellular uptake; in the small intestine, magnesium absorption partly occurs by an electrochemical gradient and by the solvent drag. A small fraction of magnesium is transported via the specific ion channels, the transient receptor potential mela- statin (TRPM) subfamily, mainly TRPM6 and TRPM7.23,40 These ion channels are assumed to be distinctive transporters for magnesium, which possess a channel and a kinase domain, and are believed to actively regulate magnesium homeostasis at the cellular level.40,41
Renal regulation of magnesium is partially achieved by reabsorption and urinary excretion (Figure 1) 23,40,42,43;
- almost 60% of filtered magnesium is reabsorbed in the cortical thick ascending limb, and
- nearly 5% to 10% is reabsorbed in the distal convoluted tubule.44
The passive paracellular reuptake of magnesium in the thick ascending limb is impaired by the mutations in claudin-16/paracellin-1, as noted in familial hypomagnesemia with hypercalciuria and nephro- calcinosis.23,45 The active transcellular transport of magnesium in the distal convoluted tubule is similarly affected by the defects in TRPM6, causing hypomagnesemia with secondary hypocalcemia.46 This channel controls the apical entry of magnesium into the tubular epithelium and changes total-body magnesium homeostasis by altering urinary excretion.
Figure 1. Factors affecting renal reabsorption of magnesium.14,23
Acid-base status
Antiduretic hormone
Calcitonin Diuretics
Glomerular filtration rate
Hypercalcemia
Overall body magnesium status
Phosphate depletion
The transcriptional activity of TRPM6 is regulated by acid-base status, 17P-estradiol, and certain immunosuppressive drugs (eg, FK506 and cyclosporine).23 Slc41a3, which is expressed in the distal convoluted tubule and the intestine, has been shown to be actively involved in systemic regulation of magnesium homeostasis.47 Genetically ablating Slc41a3 from mice has been found to induce hypomagnesemia, suggesting a role in its metabolism.
Sources of Magnesium and Vitamin D
Magnesium is naturally found in many foods, is available as a dietary supplement, and is present in such medicines as antacids and laxatives. The magnesium consumption from natural foods has decreased in the past few decades, owing to industrialized agriculture and changes in dietary habits. The standard diet in the United States contains about 50% of the recommended daily allowance (RDA) for magnesium, and as much as three-quarters of the total population is estimated to be consuming a magnesium-deficient diet.23,48 The recommended daily allowance (RDA) of magnesium for adults is 310 to 420 mg/d (Table).49 However, the required amount increases during pregnancy. It is estimated that more than 50% of women of a reproductive age do not consume the RDA for magnesium.50,51
Also, regular strenuous exercise can induce magnesium loss through urine and sweat.14 According to the 2005-2006 National Health and Nutrition Examination Survey (NHANES) data, the consumption of magnesium was below the estimated average requirement in diets of 48% of people in the United States. 50,52,53 Foods high in magnesium include almonds, bananas, beans, broccoli, brown rice, cashews, egg yolk, fish oil, flaxseed, green vegetables, milk, mushrooms, other nuts, oatmeal, pumpkin seeds, sesame seeds, soybeans, sunflower seeds, sweet corn, tofu, and whole grains. However, it is estimated that the **magnesium content in various food and vegetables is declining, ranging from 25% to 80%)) compared with the levels before 1950. 54
It may be possible that errors or differences in measurement systems due to technological advancement might show such changes in magnesium content. However, other reasons for reduced magnesium content are related to the
removal of magnesium during food processing, as well as
changes in soil conditions.
For instance, refined oils, grains, and sugar lose most of their magnesium during processing. Also, increased use of pesticides and fertilizers change soil qualities, which reduce the content of magnesium and other minerals while growing crops and vegetables.
Moreover,* changes in dietary habits from whole food without preservatives to processed fast food has also added to the reduced magnesium intake. In the Women’s Health Initiative Observational Study of 73,684 postmenopausal women, the baseline hip bone mineral density was 3% higher (and the whole-body bone mineral density was 2% higher) in women who consumed more than 422 mg/d of magnesium compared with women who consumed less than 206 mg/d.55 However, the incidence and relative risk of hip and total fractures did not differ across quintiles of magnesium intake. 55 The 2011-2012 US Department of Agriculture survey reported that the average magnesium intake for men in the United States was found to be below the RDA.56 Table. Recommended Daily Allowance of Magnesium 49 *** ||Age| Male| Female| Pregnancy
<6 mo| 30 mg| 30 mg
7-12 mo| 75 mg| 75 mg
1-3 y| 80 mg| 80 mg
4-8 y| 130 mg| 130 mg
9-13 y| 240 mg| 240 mg
14-18 y| 410 mg| 360 mga| 400 mg
19-30 y| 400 mg| 310 mga| 350 mg
31-50 y| 420 mg| 320 mga| 360 mg
51 y| 420 mg| 320 mg|| **a Recommended daily allowance for females who are not pregnant and for females who are lactating.
Although the mean magnesium intake had increased from 1977 to 2011 by approximately 15% to 357 mg/d for men,56 it was still less than the RDA of 420 mg/d.
In a comparative study of the UK government’s Composition of Food Tables, a steady decline in magnesium content was noted in commonly consumed food. For instance, between 1940 and 1991, the decline in magnesium was approximately 24% in vegetables, 17% in fruits, 15% in meats, and 26% in cheeses.57 Water is also a useful source of magnesium, with some hard tap water containing more magnesium than soft water.58 Magnesium status is low in populations who consume processed foods that are high in refined grains, fat, phosphate, and sugar.57
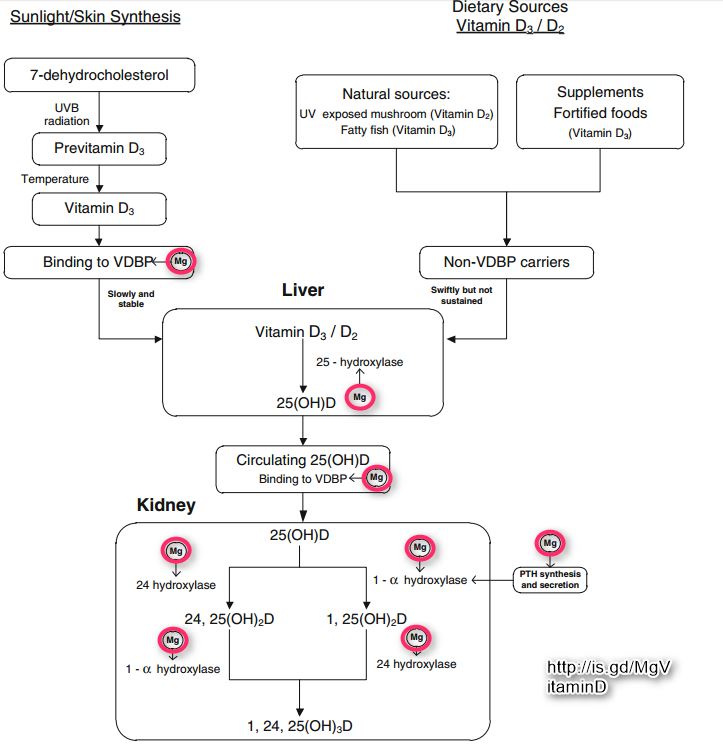
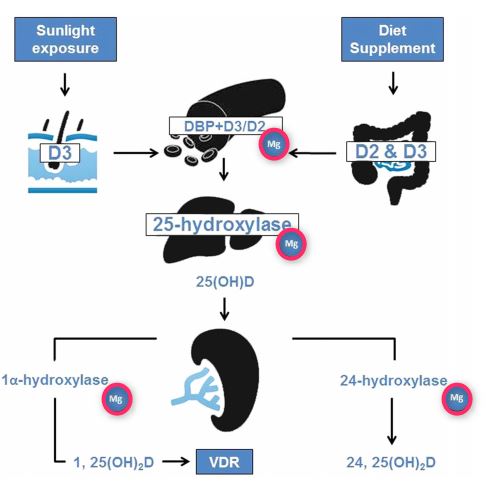
Vitamin D3 (cholecalciferol) is produced in the skin when exposed to sunlight. Vitamin D is therefore not a real vitamin. People with optimal sunlight exposure do not need to consume dietary supplementation. Because standard diets usually do not contain enough vitamin D, safe sunlight exposure or consumption of foodstuffs artificially supplemented with vitamin D are necessary to avert complications related to vitamin D deficiency.30,32,59,60 Vitamin D, either D3 (animal source) or D2 (nonanimal source), does not have significant biological activity. Rather, it needs to be processed further in the liver and kidneys to generate the biologically active form 1,25-dihydroxyvitamin D (1,25[OH]2D). This activation process occurs in 2 steps: (1) within the liver, cholecalciferol is hydroxy- lated to 25-hydroxycholecalciferol (25[OH]2D) by the enzyme 25-hydroxylase; and (2) within the kidneys, 25-hydroxycholecalciferol is converted to 1,25(OH)2D by the enzyme 1a hydroxylase.1,2,32,38,61 The enzymatic activity of both hepatic 25-hydroxylase and renal 1 a-hydroxylase is a magnesium-dependent process. Vitamin D is transported in blood bound to the carrier proteins, and the major carrier is vitamin D-binding protein. Importantly, the activity of vitamin D-binding protein is also a magnesium-dependent process (Figure 2).62'63
The high prevalence of vitamin D deficiency is a pressing global health concern, as hypovitaminosis D is claimed to be an independent risk factor for overall mor- tality.53,64 Nutrient deficiencies could be cumulative effects of dietary inadequacy, reduced absorption, or excessive excretion. Vitamin D deficiency (<12 ng/mL) can appear when regular consumption is lower than the recommended levels for a prolonged period, contact to sunlight is minimal, the kidneys are not able to generate the active form of vitamin D, or intestinal absorption of vitamin D is impaired. Vitamin D insufficiency (12-20 ng/mL) is attributed to low sunlight exposure, the source of UVB, which is required to induce vitamin D synthesis in the skin. In addition, seasonal variations, weather conditions, latitude, and clothing can influence plasma levels of 25(OH)D28,65-67; race, skin pigmentation, and age can also influence vitamin D levels. 30,68,69
Interactions Between Magnesium and Vitamin D
Nutrients interact in a coordinated manner in the body; it has been reported that 1,25(OH)2D can stimulate intestinal magnesium absorption.14 The effects of vitamin D supplementation on circulating levels of magnesium were investigated in patients with type 2 diabetes mellitus.31 In 126 adult patients with controlled diabetes (55 men and 71 women; mean [SD] age, 53.6 [10.7] years), a significant increase in serum levels of magnesium was found after they consumed vitamin D3 supplements (2000 IU/d) for 6 months.31 Conversely, magnesium acts as a cofactor for the vitamin D-binding protein. Moreover, as mentioned, the metabolism of vitamin D by hepatic 25-hydroxylation and renal 1a-hydroxylation into the active form of 1,25(OH)2D is a magnesium-dependent process. Magnesium deficiency results in reduced levels of 1,25 (OH)2D and impaired parathyroid hormone (PTH) response, and it has been implicated in magnesium- dependent vitamin D-resistant rickets.14,70,71 Magnesium supplementation was shown to markedly reduce the resistance to vitamin D treatment.14,70,71 Magnesium is the second most abundant intracellular cation and plays a key role in bone mineralization by influencing the synthesis of the active vitamin D metabolites.33,34 Studies have shown that hypovitaminosis D-associated risk of mortality could be modified by the consumption of magnesium.14,70-72 The effectiveness and clinical benefits of vitamin D are significantly reduced when magnesium homeostasis in the body is not maintained. Vitamin D also plays a key role in the intestinal absorption of phosphate and magnesium to influence eventual skeletal mineralization process.1,2,39 Earlier studies have shown that the activities of 3 major vitamin D-converting enzymes and vitamin D-binding proteins are magnesium dependent; those 3 enzymes are 25-hydroxylase in the liver and 1a- hydroxylase and 24-hydroxylase in the kidneys.33,34 Magnesium supplementation markedly reversed the resistance to vitamin D treatment in patients with rickets.14,70,71 According to the NHANES data, a high consumption of magnesium reduced the risks of vitamin D deficiency or insufficiency in the general population.53* Figure 3. Commonly encountered features of magnesium deficiency in the clinical setting.14,23 *Electrolyte Disturbance
Hypocalcemia
Hypokalemia
Neuromuscular and Central Nervous System
Athetoid movements & choreiform movements
Carpopedal spasm
Convulsions
Depression, psychosis
Muscle cramps
Muscle weakness, tremors
Nystagmus
Vertigo
Cardiovascular System
- Atrial tachycardia, fibrillation Digoxin sensitivity Supraventricular arrhythmias Ventricular arrhythmias
Complications of Magnesium Deficiency
- Altered glucose homeostasis Atherosclerotic vascular disease Hypertension Myocardial infarction Osteoporosis
Miscellaneous
Asthma
Chronic fatigue syndrome Impaired athletic performance Migraine
Also, magnesium plays a significant role in the immunoregulation of the body. It is critical to immunocompetence and in natural and adaptive immunity, partly by influencing the activity of vitamin D metabolites.22,73
Furthermore, the potential associations of serum 25 (OH)D with mortality, particularly due to cardiovascular diseases and colorectal cancer, were found to be modified by magnesium ingestion, and the inverse associations were primarily found among individuals whose magnesium intake was above the median.
Magnesium is vital for maintaining a healthy heart; it helps stabilize the rhythm of the heart and plays a role in preventing abnormal blood clotting in the heart. Magnesium also helps maintain healthy blood pressure levels.23,74,75 Studies have found that magnesium is highly effective in reducing the rate of heart attacks and strokes.14,76,77 A positive association has been found between dietary magnesium intake and bone mineral density.55,78-81 Although most osteoporosis treatment and prevention research has been centered around increased calcium and vitamin D intake, a study82 has shown that persons who consumed the highest amount of magnesium (420 mg for males and 320 mg for females) had higher bone density and lower risk of osteoporosis (Figure 3). In a study conducted on a small number of osteoporotic postmenopausal women, biochemical features of suppressed bone turnover were seen in women who consumed oral magnesium citrate for 30 days.82 Compared with baseline, serum osteocalcin levels decreased by 5% in the women who did not receive magnesium supplements (control), and serum osteocalcin levels increased by approximately 44% in women who received oral magnesium supplements. Urine deoxypyridinoline levels decreased by about 41% in the magnesium- supplemented group and by 5% in the control group (without supplements). Serum PTH levels decreased by 32% in the magnesium-supplemented group compared with 4% in the control group.82
Consuming the RDA of magnesium* may be more effective in preventing bone thinning than vitamin D , as magnesium potentiates vitamin D activities, possibly by increasing its absorption and endogenous activation 55,78-80. In bone, magnesium binds at the surface of the hydroxyapatite crystals to determine its size.83 Crystals in magnesium-deficient bones are bigger, and they may form brittle bones that are prone to fractures. *84 In addition to skeletal mineralization, magnesium also helps in osteoblast proliferation, and its deficiency impairs bone formation.85 Magnesium-deficient rats have decreased bone mass related to reduced numbers of osteoblasts.86,87
Magnesium has been found to be a contributing factor in patients with established osteoporosis with vitamin D deficiency and blunted PTH level.88 Studies have suggested that magnesium could influence PTH synthesis and* determine the number of vitamin D receptor *s; therefore, a deficiency in magnesium levels may lead to diminished synthesis and secretion of PTH and a reduced number of available vitamin D receptors in the target cells.42 One study 53 claimed that a significant increase in serum 25(OH)D was achieved only when vitamin D supplementation was given with magnesium; another study 89 concurred, finding no increase in serum 25(OH)D level either with vitamin D or magnesium supplementation alone. A study in mice showed that magnesium deficiency during pregnancy influences both maternal and fetal fatty acid metabolism and adversely affects fetal growth and survival, emphasizing the importance of adequate maternal magnesium status for better pregnancy outcome.90
Future Research
Magnesium is an essential cofactor for vitamin D synthesis, and activated vitamin D, in turn, can increase intestinal absorption of magnesium and, therefore, can form a feed-forward loop to maintain its homeostasis. With regard to the musculoskeletal system, future study may explore the synergistic effect of vitamin D and magnesium levels along with osteopathic manipulative treatment on performance. The roles and regulation of magnesium in health and diseases are a rapidly evolving area. Studies have shown that magnesium supplementation can increase the effectiveness of vitamin D activity; therefore, further controlled studies should determine the dose of magnesium required for a particular clinical situation for reducing vitamin D-asso- ciated disorders.
Conclusion
Magnesium homeostasis is maintained by the delicate interactions of the intestine, bone, and kidneys.
Magnesium is an essential cofactor for vitamin D synthesis and activation and, in turn, can increase intestinal absorption of magnesium and establish a feed-forward loop to maintain its homeostasis. Dysregulation in either of these nutrients can be associated with various disorders, including skeletal deformities, cardiovascular disorders, and metabolic syndrome.91 A core principle of osteopathic medicine lies in promoting the body’s innate ability to heal itself. A better understanding of how magnesium supplementation might reduce complications related to vitamin D deficiency would help improve patient care.
References
Dusso AS. Update on the biologic role of the vitamin D endocrine system. Curr Vasc Pharmacol. 2014;12(2):272-277. doi:10.2174/15701611113119990026
Brown RB, Haq A, Stanford CF, Razzaque MS. Vitamin D, phosphate, and vasculotoxicity. Can J Physiol Pharmacol. 2015;93 (12):1077-1082. doi:10.1139/cjpp-2015-0083
Razzaque MS. Bone-kidney axis in systemic phosphate turnover. Arch Biochem Biophys. 2014;561:154-158. doi:10.1016/j.abb.2014.06.031
Razzaque MS. Phosphate toxicity: new insights into an old problem. Clin Sci (Lond). 2011;120(3):91-97. doi:10.1042/CS20100377
Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5(11):611-619. doi:10.1038/nrendo.2009.196
Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am J Physiol Renal Physiol. 2009;296(3):F470-476. doi:10.1152/ajprenal.90538.2008
Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. 2012;5(suppl 1):i3-i14. doi:10.1093/ndtplus/sfr163
Meintzer RB, Steenbock H. Vitamin D and magnesium absorption.J Nutr. 1955;56(2):285-294.
Lynch HT, Lemon HM, Henn MJ, Ellingson RJ, Grissom RL. Vitamin D-intoxicated patient with hypoparathyroidism; hypercalcemia, acute cerebellar ataxia, and eeg changes: magnesium sulfate therapy. Arch Intern Med. 1964;114:375-380. doi:10.1001 /archinte.1964.03860090109011
Reddy P, Edwards LR.** Magnesium supplementation in vitamin D deficiency__. Am J Ther. 2017. doi:10.1097/MJT.0000000000000538
- See Vitamin D Life Vitamin D supplementation often needs Magnesium – May 2017
Nellis JC, Tufano RP, Gourin CG. Association between magnesium disorders and hypocalcemia following thyroidectomy. OtolaryngolHead NeckSurg. 2016;155(3):402-410. doi:10.1177/0194599816644594
Haq A, Svobodova J, Imran S, Stanford C, Razzaque MS. Vitamin D deficiency: a single centre analysis of patients from 136 countries. J SteroidBiochem MolBiol. 2016;164:209-213. doi:10.1016/j. jsbmb.2016.02.007
Caspi R, Altman T, Dreher K, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2012;40(database issue):D742-D753. doi:10.1093/nar/gkx935
Swaminathan R. Magnesium metabolism and its disorders. Clin Biochem Rev. 2003;24(2):47-66.
Noronha JL, Matuschak GM. Magnesium in critical illness: metabolism, assessment, and treatment. Intensive Care Med. 2002;28 (6):667-679. doi:10.1007/s00134-002-1281-y
Houillier P. Mechanisms and regulation of renal magnesium transport. Annu Rev Physiol. 2014;76:411-430. doi:10.1146 /annurev-physiol-021113-170336
Chamnongpol S, Groisman EA. Mg2+ homeostasis and avoidance of metal toxicity. Mol Microbiol. 2002;44(2):561-571. doi:10.1046 /j.1365-2958.2002.02917.x
Weglicki WB, Mak Iu T, Chmielinska JJ, Tejero-Taldo MI, Komarov AM, Kramer JH. The role of magnesium deficiency in cardiovascular and intestinal inflammation. Magnes Res. 2010;23(4):S199-206. doi:10.1684/mrh.2010.0218
Kramer JH, Mak IT, Phillips TM, Weglicki WB. Dietary magnesium intake influences circulating pro-inflammatory neuropeptide levels and loss of myocardial tolerance to postischemic stress. Exp Biol Med (Maywood). 2003;228(6):665-673.
de Rouffignac C, Quamme G. Renal magnesium handling and its hormonal control. Physiol Rev. 1994;74(2):305-322. doi:10.1152 /physrev.1994.74.2.305
Quamme GA, de Rouffignac C. Epithelial magnesium transport and regulation by the kidney. Front Biosci. 2000;5:D694-D711.
Touyz RM. Magnesium in clinical medicine. Front Biosci. 2004;9:1278-1293.
Seo JW, Park TJ. Magnesium metabolism. Electrolyte Blood Press. 2008;6(2):86-95. doi:10.5049/EBP.2008.6.2.86
Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010;23(4):S194-S198. doi:10.1684/mrh.2010.0213
Welsh J. Function of the vitamin D endocrine system in mammary gland and breast cancer. Mol Cell Endocrinol. 2017;453:88-95. doi:10.1016/j.mce.2017.04.026
Chirumbolo S, Bjorklund G, Sboarina A, Vella A. The role of vitamin D in the immune system as a pro-survival molecule. Clin Ther. 2017;39 (5):894-916. doi:10.1016/j.clinthera.2017.03.021
Berridge MJ. Vitamin D deficiency and diabetes. Biochem J. 2017;474 (8):1321-1332. doi:10.1042/BCJ20170042
Uwitonze AM, Murererehe J, Ineza MC, et al. Effects ofvitamin D status on oral health. J Steroid Biochem Mol Biol. 2017;175:190-194. doi:10.1016/j.jsbmb.2017.01.020
Haq A, Svobodova J, Sofi NY, et al. Vitamin D status among the juvenile population: a retrospective study. J Steroid Biochem Mol Biol. 2017;175:49-54. doi:10.1016/j.jsbmb.2017.01.005
Razzaque MS. Sunlight exposure: do health benefits outweigh harm? J SteroidBiochem. MolBiol. 2018;175:44-48. doi:10.1016 /j.jsbmb.2016.09.004
Al-Daghri NM, Alkharfy KM, Khan N, et al. Vitamin D supplementation and serum levels of magnesium and selenium in type 2 diabetes mellitus patients: gender dimorphic changes. Int J Vitam NutrRes. 2014;84(1-2):27-34. doi:10.1024/0300-9831/ a000190
Razzaque MS. The dualistic role of vitamin D in vascular calcifications. KidneyInt. 2011;79(7):708-714. doi:10.1038 /ki.2010.432
Risco F, Traba ML. Possible involvement of a magnesium dependent mitochondrial alkaline phosphatase in the regulation of the 25-hydroxyvitamin D3-1 alpha-and 25-hydroxyvitamin D3-24R-hydroxylases in LLC-PK1 cells. Magnes Res. 1994;7 (3-4):169-178.
Risco F, Traba ML. Influence of magnesium on the in vitro synthesis of 24,25-dihydroxyvitamin D3 and 1 alpha, 25-dihydroxyvitamin D3. MagnesRes. 1992;5(1):5-14.
Cerit L. Genetic variation in vitamin D receptor gene (Fok1:rs2228570) is associated with risk of coronary artery disease. Biomarkers. 2017;22 (3-4):387. doi:10.1080 /1354750X.2016.1204008
Sath S, Shah AR, Nadeem S, Rafiq SN, Jeelani I. Hypervitaminosis D in Kashmiri population: a case series of 11 patients. SSRG Int J Med Sci. 2016;3:1-6. doi:10.14445/23939117/IJMS-V3I2P101
Reynolds JA, Bruce IN. Vitamin D treatment for connective tissue diseases: hope beyond the hype? Rheumatology (Oxford). 2017;56 (2):178-186.doi:10.1093/rheumatology/kew1212
Holick MF. Vitamin D evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4-18.
Lanske B, Razzaque MS. Vitamin D and aging: old concepts and new insights. JNutr Biochem. 2007;18(12):771-777. doi:10.1016 /j.jnutbio.2007.02.002
Beggs MR, Appel I, Svenningsen P, Skjodt K, Alexander RT, Dimke H. Expression of transcellular and paracellular calcium and magnesium transport proteins in renal and intestinal epithelia during lactation. Am J Physiol Renal Physiol. 2017;313(3):F629-F640. doi:10.1152 /ajprenal.00680.2016.
Hoorn EJ, Zietse R. Disorders of calcium and magnesium balance: a physiology-based approach. Pediatr Nephrol. 2013;28(8):1195-1206. doi:10.1007/s00467-012-2350-2
Rodriguez-Ortiz ME, Canalejo A, Herencia C, et al. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptorexpression at moderately lowcalcium concentration. Nephrol Dial Transplant. 2014;29(2):282-289. doi:10.1093/ndt/gft400
Cunningham J, Rodriguez M, Messa P. Magnesium in chronic kidney disease Stages 3 and 4 and in dialysis patients. Clin KidneyJ. 2012;5 (suppl 1):i39-i51. doi:10.1093/ndtplus/sfr166
Yu AS. Evolving concepts in epithelial magnesium transport. Curr Opin Nephrol Hypertens. 2001;10(5):649-653.
Glaudemans B, Knoers NV, Hoenderop JG, Bindels RJ. New molecular players facilitating Mg(2+) reabsorption in the distal convoluted tubule. KidneyInt. 2010;77(1):17-22. doi:10.1038 /ki.2009.358
Glaudemans B, van der Wijst J, Scola RH, et al. A missense mutation in the Kv1.1 voltage-gated potassium channel-encoding gene KCNA1 is linked to human autosomal dominant hypomagnesemia. J Clin Invest. 2009;119(4):936-942. doi:10.1172/JCI36948
de Baaij JH, Arjona FJ, van den Brand M, et al. Identification of SLC41A3 as a novel player in magnesium homeostasis. Sci Rep. 2016;6:28565. doi:10.1038/srep28565
Choi YH, Miller JM, Tucker KL, Hu H, Park SK. Antioxidant vitamins and magnesium and the risk of hearing loss in the US general population. Am J Clin Nutr. 2014;99(1):148-155. doi:10.3945 /ajcn.113.068437
Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press; 2010.
Rosanoff A, Weaver CM, Rude RK. Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr Rev. 2012;70(3):153-164. doi:10.1111/j.1753-4887.2011.00465.x
Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133(9):2879-2882.
Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7 :121-134. doi:10.3945/an.115.009258
Deng X, Song Y, Manson JE, et al. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013;11:187. doi:10.1186/1741-7015-11-187
- in Vitamin D Life Magnesium increases Vitamin D and decreases mortality - 2013
Thomas D. The mineral depletion of foods available to us as a nation (1940-2002)—a review of the 6th edition of McCance and Widdowson. Nutr Health. 2007;19(1-2):21-55.
Orchard TS, Larson JC, Alghothani N, et al. Magnesium intake, bone mineral density, and fractures: results from the Women’s Health Initiative Observational Study. Am J Clin Nutr. 2014;99(4):926-933. doi:10.3945/ajcn.113.067488
Table 37: total nutrient intakes: percent reporting and mean amounts of selected vitamins and minerals from food and beverages and dietary supplements, by gender and age, in the United States, 2011-2012. United States Department of Agriculture website. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1112 /Table37SUPGEN11.pdf.
Thomas D. A study on the mineral depletion ofthe foods available to us as a nation over the period 1940 to 1991. Nutr Health. 2003;17 (1) :85-115. doi:10.1177/026010600301700201
Jiang L, He P, Chen J, et al. Magnesium levels in drinking water and coronary heart disease mortality risk: a meta-analysis. Nutrients. 2016;8(1). doi:10.3390/nu8010005
Uwitonze AM, Uwambaye P, Isyagi M, et al. Periodontal diseases and adverse pregnancy outcomes: is there a role for vitamin D [published online January 16, 2018]? J Steroid Biochem Mol Biol. doi:10.1016 /j.jsbmb.2018.01.010
Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8-28. doi:10.1152/ajprenal.00336.2004
Holick MF. Sunlight, ultraviolet radiation, vitamin D and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1-16.
Rude RK. Skeletal adenylate cyclase: effect of Mg2+, Ca2+, and PTH. CalcifTissue Int. 1985;37(3):318-323.
Rude RK, Adams JS, Ryzen E, et al. Low serum concentrations of 1,25-dihydroxyvitamin D in human magnesium deficiency. J Clin EndocrinolMetab. 1985;61(5):933-940. doi:10.1210 /jcem-61-5-933
Nair R, Maseeh A. Vitamin D: the "sunshine" vitamin. J Pharmacol Pharmacother. 2012;3(2):118-126. doi:10.4103/0976-500X.95506
Holick MF. Deficiency of sunlight and vitamin D. BMJ. 2008;336 (7657):1318-1319. doi:10.1136/bmj.39581.411424.80
Holick MF. Sunlight, UV-radiation, vitamin D and skin cancer: how much sunlight do we need? AdvExp Med Biol. 2008;624:1-15. doi:10.1007/978-0-387-77574-6_1
Saraff V, Shaw N. Sunshine and vitamin D. Arch Dis Child. 2016;101 (2):190-192. doi:10.1136/archdischild-2014-307214
Holick MF. Vitamin D and sunlight: strategies for cancer prevention and other health benefits. Clin J Am Soc Nephrol. 2008;3 (5):1548-1554. doi:10.2215/CJN.01350308
Razzaque MS. Can adverse effects of excessive vitamin D supplementation occur without developing hypervitaminosis D? J Steroid Biochem Mol Biol. 2017;pii:S0960-0760(17)30171-1. doi:10.1016/j.jsbmb.2017.07.006
Ozsoylu S, Hanioglu N. Serum magnesium levels in children with vitamin D deficiency rickets. Turk JPediatr. 1977;19(3-4):89-96.
Anast CS. Magnesium studies in relation tovitamin D-resistant rickets. Pediatrics. 1967;40(3):425-435.
Medalle R, Waterhouse C, Hahn TJ. Vitamin D resistance in magnesium deficiency. Am J Clin Nutr. 1976;29(8):854-858.
Tam M, Gomez S, Gonzalez-Gross M, Marcos A. Possible roles of magnesium on the immune system. Eur J Clin Nutr. 2003;57 (10):1193-1197. doi:10.1038/sj.ejcn.1601689
Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr. 2012;66(4): 411-418. doi:10.1038/ejcn.2012.4
Grober U, Schmidt J, Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7(9):8199-8226. doi:10.3390/nu7095388
Joao Matias P, Azevedo A, Laranjinha I, et al. Lower serum magnesium is associated with cardiovascular risk factors and mortality in haemodialysis patients. Blood Purif. 2014;38(3-4):244-252. doi:10.1159/000366124
Song Y, Manson JE, Cook NR, Albert CM, Buring JE, Liu S. Dietary magnesium intake and risk of cardiovascular disease among women. Am J Cardiol. 2005;96(8):1135-1141. doi:10.1016 /j.amjcard.2005.06.045
Farsinejad-Marj M, Saneei P, Esmaillzadeh A. Dietary magnesium intake, bone mineral density and risk offracture: a systematic review and meta-analysis. Osteoporos Int. 2016;27(4):1389-1399. doi:10.1007/s00198-015-3400-y
Nieves JW. Bone. Maximizing bone health—magnesium, BMD and fractures. Nat Rev Endocrinol. 2014;10(5):255-256. doi:10.1038 /nrendo.2014.39
Yoshizawa S, Brown A, Barchowsky A, SfeirC. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10(6):2834-2842. doi:10.1016/j.actbio.2014.02.002
Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr. 2009;28(2):131-141.
Aydin H, Deyneli O, Yavuz D, et al. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporoticwomen. Biol Trace Elem Res. 2010;133(2):136-143. doi:10.1007/s12011-009-8416-8
Salimi MH, Heughebaert JC, Nancollas GH. Crystal growth of calcium phosphates in the presence of magnesium ions. Langmuir. 1985;1:119-122. doi:10.1021/la00061a019
Cohen L, Laor A, Kitzes R. Bone magnesium, crystallinity index and state of body magnesium in subjects with senile osteoporosis, maturity-onset diabetes and women treated with contraceptive preparations. Magnesium. 1983;2:70-75.
Lu WC, Pringa E, Chou L. Effect of magnesium on the osteogenesis of normal human osteoblasts. Magnes Res. 2017;30(2):42-52. doi:10.1684/mrh.2017.0422
Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: animal and human observations. J NutrBiochem. 2004;15(12):710-716. doi:10.1016/j.jnutbio.2004.08.001
Rude RK, Gruber HE, Norton HJ, Wei LY, Frausto A, Mills BG. Bone loss induced by dietary magnesium reduction to 10% of the nutrient requirement in rats is associated with increased release of substance P and tumor necrosis factor-alpha. J Nutr. 2004;134 (1):79-85.
Sahota O, Mundey MK, San P, Godber IM, Hosking DJ. Vitamin D insufficiency and the blunted PTH response in established osteoporosis: the role of magnesium deficiency. Osteoporos Int. 2006;17(7):1013-1021. doi:10.1007/s00198-006-0084-3
Fuss M, Bergmann P, Bergans A, et al. Correction of low circulating levels of 1,25-dihydroxyvitamin D by 25-hydroxyvitamin D during reversal of hypomagnesaemia. Clin Endocrinol (Oxf). 1989;31 (1):31-38.
Gupta M, Solanki MH, Chatterjee PK, et al. Maternal magnesium deficiency in mice leads to maternal metabolic dysfunction and altered lipid metabolism with fetal growth restriction. Mol Med. 2014;20:332-340. doi:10.2119/ molmed.2014.00137
Moore-Schiltz L, Albert JM, Singer ME, Swain J, Nock NL. Dietary intake of calcium and magnesium and the metabolic syndrome in the National Health and Nutrition Examination (NHANES) 2001-2010 data. Br J Nutr. 2015;114(6):924-935. doi:10.1017 /S0007114515002482
