Hypothesis – Vitamin D help MicroRNAs reduce Virus Infection
Vitamin D-Regulated MicroRNAs: Are They Protective Factors against Dengue Virus Infection?
Hindawi Publishing Corporation Advances in Virology. Volume 2016, Article ID 1016840, 14 pages http://dx.doi.org/10.1155/2016/1016840
John F. Arboleda and Silvio Urcuqui-Inchima
Grupo Inmunovirologia, Facultad de Medicina, Universidad de Antioquia (UdeA), Calle 70 No. 52-51, Medellin, Colombia Correspondence should be addressed to Silvio Urcuqui-Inchima; [email protected]
Over the last few years, an increasing body of evidence has highlighted the critical participation of vitamin D in the regulation of proinflammatory responses and protection against many infectious pathogens, including viruses. The activity of vitamin D is associated with microRNAs, which are fine tuners of immune activation pathways and provide novel mechanisms to avoid the damage that arises from excessive inflammatory responses. Severe symptoms of an ongoing dengue virus infection and disease are strongly related to highly altered production of proinflammatory mediators, suggesting impairment in homeostatic mechanisms that control the host’s immune response. Here, we discuss the possible implications of emerging studies anticipating the biological effects of vitamin D and microRNAs during the inflammatory response, and we attempt to extrapolate these findings to dengue virus infection and to their potential use for disease management strategies.
📄 Download the PDF from Vitamin D Life
Introduction
Activation of innate immune cells results in the release of proinflammatory mediators to initiate a protective local response against invading pathogens [1]. However, overactivated inflammatory activity could be detrimental since it can cause tissue damage and even death of the host. Therefore, negative feedback mechanisms are required to control the duration and intensity of the inflammatory response [1, 2]. Although little is known about the molecular mechanisms occurring during dengue virus (DENV) infection/disease, it has been suggested that the immune response initiated against the virus greatly contributes to pathogenesis. Indeed, several symptoms of the disease are tightly related to imbalanced immune responses, particularly to high production of proinflammatory cytokines [3, 4] suggesting an impairment of homeostatic mechanisms that control inflammation. Interestingly, vitamin D has been described as an important modulator of immune responses to several pathogens and as a key factor enhancing immunoregulatory mechanisms that avoid the damage that arises from excessive inflammatory responses [5, 6], as in dengue disease [7]. Mounting evidence obtained from human populations and experimental in vitro studies has suggested that this hormone can play a key role in the immune system’s response to several viruses [814], thereby becoming a potential target of intervention to combat DENV infection and disease progression. Among several mechanisms, vitamin D activity has been associated with the expression of certain microRNAs (miRs) [15] that are one of the main regulatory switches operating at the translational level [16]. miRs constitute approximately 1% of the human genome and their sequences can be found within introns of other genes or can be encoded independently and transcribed in a similar fashion to mRNAs encoded by protein-coding genes [16]. A typical mature miR of 18-23 base pairs associates with the RNA-induced silencing complex (RISC) and moves towards the target mRNA [17]. Once there, the miR binds to the complementary sequence in the 3,untranslated region (37UTR) of the mRNA, thereby inducing gene silencing through mRNA cleavage, translational repression, or deadenylation [16]. A single miR may directly regulate the expression of hundreds of mRNAs at once and several miRs can also target the same mRNA resulting in enhanced translation inhibition [18]. Targeting of specific genes involved in modulation of immune response pathways by miRs provides a finely tuned regulatory mechanism for the restoration of the host’s resting inflammation state [1921]. Since the association between vitamin D and miR activity may play a relevant role in ongoing DENV infections, here we provide an overview of DENV-induced inflammatory responses and the early evidence anticipating a possible participation of the vitamin D and miR interplay regulating antiviral and inflammatory responses during DENV infection/disease.
DENV and the Immune Response
DENV is an icosahedral-enveloped virus with a positive sense single-stranded RNA (ssRNA) genome that belongs to the family Flaviviridae, genus Flavivirus. There are four phylogenetically related but antigenically distinct viral serotypes (DENV 1-4) able to cause the full spectrum of the disease [22]. In addition, a sylvatic serotype (DENV-5), with no evidence regarding its ability to infect humans, has been recently reported [23]. DENV is transmitted by Aedes mosquitoes in tropical and subtropical areas where the disease has become a major public health threat and one of the most rapidly spreading vector-borne diseases in the world, with an increasing incidence of 30-fold in the past 50 years [24, 25]. An estimated 3.6 billion people live in high risk areas worldwide and it is estimated that over 390 million cases occur every year , of which 96 million suffer from dengue fever [26-28]. Although only a minor number of cases may progress to the severe forms of the disease, 21.000 deaths are reported annually [27]. Guidelines of the World Health Organization (WHO) recognize dengue as a clinical continuum from dengue fever (DF), a nonspecific febrile illness, to dengue with or without warning signs that can progress to dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) [3]. These severe forms of the disease are characterized by a wide spectrum of symptoms, including the development of vascular permeability, plasma leakage, thrombocytopenia, focal or generalized hemorrhages, and tissue and/or organ damage that may lead to shock and death [29, 30]. Besides ecoepidemiology, host genetic variations, and virus virulence, the risk factor is increased mainly by secondary infections with different dengue serotypes, presumably through a mechanism known as antibody-dependent immune enhancement (ADE), whereby nonneutralizing antibodies from previous heterotypic infections enhance virus entry via receptors for immunoglobulins or Fc receptors (FcRs) [29, 31, 32].
Skin is the first barrier for the invading DENV and the site where innate immunity exerts the first line of defense [33]. Following the bite by an infected mosquito, local tissue resident dendritic cells (DCs) and macrophages are the main targets of the virus [34, 35]. The viral structural E protein binds to cellular receptors, such as DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule-3- Grabbing Nonintegrin), CLEC5A (C-type lectin domain family 5, member A), and MR (mannose receptor), allowing internalization of the virus through receptor-mediated endocytosis [22, 36-38]. Once in the cytoplasm, DENV replication products, such as double-stranded RNA (dsRNA) or genomic ssRNA, are sensed by several pattern recognition receptors (PRRs) (Figure 1), including TLR3, TLR7, TLR8, the cytosolic receptors RIG-I (Retinoic acid Inducible Gene-1), and MDA-5 (Melanoma Differentiation-Associated protein 5) [39-43]. Subsequently, this subset of PRRs triggers the activation of intracellular pathways, leading to the activation of transcription factors such as interferon regulatory factors 3 and 7 (IRF3 and IRF7) and the Nuclear Factor kB (NF- kB) and the later production of type I interferons and proinflammatory cytokines promoting an antiviral response [44, 45]. Additionally, the local activation of natural killer (NK) cells, neutrophils, and mast cells by the presence of the virus induces more proinflammatory mediators, complement activation, and the commitment of cellular and humoral immune responses to clear and control viral infection [46].
Figure 1: Potential link between vitamin D and miR controlling DENV-induced inflammatory response and antiviral activity. (1) DENV replication products and proteins are recognized by several PRRs whose signaling pathways promote the proinflammatory response. (2) Vitamin D activity induces transcription of microR- NAs and other target genes that play a critical role in the control of inflammation-related signaling pathways and antiviral activity.
Inflammation and Cytokine Storm . Although the immune response is critical to combat and overcome invading pathogens, it is believed that the immune response greatly contributes to progression of dengue disease [31]. The pathogenesis and progression to the severe forms of dengue are still not completely understood; however, most cases are characterized by bleeding, hemorrhage, and plasma leakage that can progress to shock or organ failure [87, 88]. These physiological events are preceded by a hyperpermeability syndrome caused mainly by an imbalance between proinflammatory and anti-inflammatory cytokines produced in response to virus infection. The predominant proinflammatory mediators or “cytokine storm,” secreted mainly by T cells, monocytes/macrophages, and endothelial cells
Table 1
Regardless of global serum vitamin D levels, sensing of microbial pathogens via PRRs induces upregulation of CYP27B1 and, as a consequence, local conversion of 1,25(OH)2D3 from 25(OH)D3, enhancing VDR nuclear translocation and subsequent transcription of target genes to exert antimicrobial effects [113, 117— 119]. This establishes a linkage between vitamin D status and the intracrine and paracrine modulation of cellular immune responses, in which VDR and CYP27B1 activity are of central importance [117, 118, 120]. Indeed, this link is also evidenced by studies in which pathogen susceptibility associated with vitamin D deficiency/insufficiency levels is reduced by correct supplementation [121,122]. Furthermore, some vitamin D-induced antiviral mechanisms have been shown by preliminary reports (Table 2). Peptides such as cathelicidins are strongly upregulated by 1,25(OH)2D3 due to its VDR response elements. In humans, active cathelicidin is known as LL-37 and has a C-terminal cationic antimicrobial domain that can induce bacterial membrane disruption and inhibition of herpes simplex virus, influenza virus, and retroviral replication, among others [55-57]. In fact, very recent reports have suggested an association between vitamin D and the LL-37 antiviral activity to HIV and rhinovirus [58, 59]. Likewise, HBD-2 is also induced by 1,25(OH)2D3. Interestingly, a correlation between VDR and HBD-2 was found to be associated with natural resistance to HIV infection, suggesting the potential participation of vitamin D- induced resistance to the virus [60,106]. Moreover, vitamin D can also induce reactive oxygen species (ROS) that associates with suppression of the replicative activity of some viruses, such as hepatitis C virus (HCV) [61]. Although the vitamin D-induced antiviral mechanisms are not fully elucidated and further studies are needed to fully understand their roles, many are possible due to the pleiotropic nature of vitamin D and the complex transcriptional modulation of hundreds of genes controlled by its activity.
Several studies have reported a link between VDR polymorphisms and severe outcomes of bronchiolitis and acute lower respiratory tract infections (RTIs) with respiratory syncytial virus (RSV) [105]. Indeed, vitamin D supplementation is associated with reduced RTI, vitamin D status, and serum concentrations in children [123]. Likewise, some vitamin D supplementation studies have reported a reduction in cold/influenza linked to seasonal sunlight exposure and skin pigmentation [124]. In HIV infection, associations have also been reported between vitamin D levels with progression of the disease, survival times of HIV patients, CD4+ T cell counts, inflammatory responses, and potential impact of HAART (Highly Active Anti-Retroviral Therapy) treatments [125]. Finally, similar population and ecoepidemiological reports have associated the role of vitamin D in several viral infections, including DENV and other flaviviruses [10-13], not only highlighting inhibition of viral replication but also controlling the inflammatory response and progression of the disease.
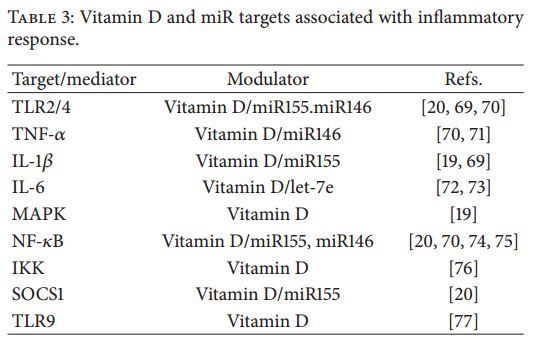
In addition to viral control, vitamin D-induced immune mechanisms have important effects providing potential feedback modulation in pathways that regulate immune activation, avoiding excessive elaboration of the inflammatory responses and its potential risk for tissue homeostasis (Table 3) [5, 6, 126]. TLRs can both affect and be affected by VDR signaling and likewise some antimicrobial peptides associated with TLRs have demonstrated antiviral effects [6, 13, 127]. In this sense, and due to the interest in the modulatory effect of vitamin D on TLR expression and proinflammatory cytokine production, some authors have shown that vitamin D can induce hyporesponsiveness to PAMPs (Pathogen-Associated Molecular Patterns) by downregulating the expression of TLR2 and TLR4 on monocytes that in turn have been associated with impaired production of TNF-a, suggesting a critical role of vitamin D in regulating TLR driven inflammation [71]. Importantly, a link between the DENV NS1 protein and activation of the inflammatory response via TLR2 and TLR4 impacting the progression of the disease has very recently been described [93,128]. DENV NS1 antigens may induce the activation of TLR2 and TLR4 inducing high secretion of proinflammatory mediators that enhance endothelial dysfunction and permeability [46, 94, 129, 130]. Interestingly, it was reported that 1,25(OH)2D3 significantly reduces the levels of TLR2/TLR4 expression and of proinflammatory cytokines (TNF-a, IL-6, IL-12p70, and IL-1 ) produced by U937 cells after exposure to DENV [72]. The same approach used in primary human monocytes and macrophages led to similar results, consistent with data obtained in our laboratory [19]. It has been suggested that vitamin D may regulate proinflammatory cytokine levels by targeting TLR activation signaling molecules (Figure 1). Indeed, it has been reported that treatment of monocytes with 1,25(OH)2D3 regulates TLR expression via the NF-kB pathway and reduces signaling of the mitogen-activated protein kinases MAPKs/p38 and p42/44 [19]. One of the most critical steps in NF-kB regulation is ItcBa proteasomal degradation mediated by IKK (I kappa B Kinase) that leads to the nuclear entry of the NF-kB heterodimer p65/p50 to trans- activate gene expression, resulting in a decrease of inflammatory genes. Accordingly, a novel molecular mechanism has recently been described in which 1,25(OH)2D3 binding to VDR attenuates NF-kB activation by directly interacting with the IKK protein to block its activity and, consequently, the NF- B-dependent inflammatory response [76]. Besides TLR2 and TLR4, it has been shown that vitamin D can also downregulate the intracellular TLR9 expression and, subsequently, lead to less secretion of IL-6 in response to TLR9 stimulation [77]. Although intracellular downregulation of some PRRs such as TLR3, TLR7/8, and RIG-I/MDA5 may affect the potential antiviral response induced by type IIFN, various reports have shown that vitamin D treatment does not affect the type I IFN-induced antiviral response against various viruses [69, 131, 132]. In fact, it has been reported that porcine rotavirus (PRV) infection induces CYP27B1- dependent generation of 1,25(OH)2D3 which leads to an increased expression of TLR3 and RIG-I that consequently enhance the type I IFN-dependent antiviral response [76].
Vitamin D and miRs: Potential Implications for Inflammation Balance.
Although vitamin D may impact distinct pathways and molecules to modulate inflammatory responses, current evidence suggests TLRs and TLR signaling mediators as main targets by which vitamin D modulates inflammation (Table 3) [6, 113, 133, 134]. However, a novel regulatory vitamin D mechanism in which TLR signaling/activation and miR function are associated has been recently documented, suggesting a crucial role of vitamin D and miRs for the host immune system homeostasis [15,135,136]. The participation of miRs as general regulatory mechanisms of initiation, propagation, and resolution of immune responses has been widelyreviewedelsewhere [21,137,138]. Therefore, we discuss here its potential relationship with vitamin D activity in the control of inflammatory responses, attempting to extrapolate these findings to DENV infection.
The ability of vitamin D to regulate miRs and their emerging relationship have been proposed by means of several experimental and clinical approaches; however, the implications of their impact on inflammatory responses have only been studied in in vitro models [15, 20, 135, 136, 139]. In patient trials with vitamin D supplementation, significant differences in miR expression profiles have been reported, suggesting that dietary vitamin D may also globally regulate miR levels [15]. Although several mechanisms may be involved in regulating such a global effect, some authors have found that chromatin states may be altered by VDR activity, determining accessibility for binding of the transcription and regulation of activation or inhibition of transcription [140, 141]. This in turn could be of relevance for canonical VDR-VDRE-mediated transcription regulation. In fact, VDR-induced regulation of miRs via VDRE has been demonstrated for some miRs such as miR-182 and let- 7a whose pri-miRs (Primary miR) have multiple VDR/RXR binding sites, suggesting that these miRs could potentially be regulated by vitamin D metabolites [67, 142]. Moreover, a negative feedback loop between some miRNAs and VDR signaling has been reported. This is the case of miR-125b whose overexpression can reduce VDR/RXR protein levels. Since miR-125b is commonly downregulated in cancer cells, it has been proposed that such a decrease in miR-125b may result in the upregulation of VDR and in increasing antitumor effects driven by vitamin D in cancer cell models [136].
Additionally, it has been reported that VDR signaling may attenuate TLR-mediated inflammation by enhancing a negative feedback inhibition mechanism (Figure 1). A recent report has shown that VDR inactivation leads to a hyper- inflammatory response in LPS-cultured mice macrophages through overproduction of miR-155 which in turns down- regulates the suppressor of the cytokine signaling (SOCS) family of proteins that are key components of the negative feedback loop regulating the intensity, duration, and quality of cytokine signaling [2, 143, 144]. As feedback inhibitors of inflammation, SOCS proteins are upregulated by inflammatory cytokines, and, in turn, they block cytokine signaling by targeting the JAK/STAT (Janus Kinase/Signal Transducer and Activator of Transcription) pathway [2]. Evidence suggests that SOCS inhibits the proinflammatory pathways of cytokines such as TNF-a, IL-6, and IFN-y and can inhibit the LPS-induced inflammatory response by directly blocking TLR4 signaling by targeting the IL-1R- associated kinases (IRAK) 1 and 4 [20, 144]. Consequently, deletion of miR-155 attenuates 1,25(OH)2D3 suppression of LPS-induced inflammation, confirming that vitamin D stimulates SOCS1 by downregulating miR-155 [20]. Taken together, these results highlight the importance of the VDR pathways controlling the inflammatory response by modulating miRNA-155-SOCS1 interactions. Finally, an additional reinforcing issue that may validate the link between vitamin D activity and miRs is the fact that 1,25(OH)2D3 deficiency has been related to reduced leukotriene synthetic capacity in macrophages [145, 146]. Recently, it was reported that leukotriene B4 (LTB4) can upregulate macrophage MyD88 (Myeloid Differentiation primary response-88) expression by decreasing SOCS-1 stability that is associated with the expression of proinflammatory miRs, such as miR-155, miR- 146b, and miR-125b, and TLR4 activation in macrophages [147]. miR-146 has been also shown as a modulator of inflammatory responses mediated by TLR4/NF-kB and TNF- a [70]. Importantly, this miR has been found downregulated in patients with autoimmune disorders in which low levels of vitamin D have also been reported [148,149]. These results suggest that vitamin D can orchestrate miR diversity involved in TLR signaling, thereby regulating inflammatory responses and activation of immune responses.
Insights into Vitamin D and DENV Infection
Little is known about the link between DENV infection and vitamin D; however, since severe dengue is associated with imbalanced production of proinflammatory cytokines, it is very tempting to suggest that vitamin D could play an important role in modulating the inflammatory responses during ongoing DENV infections . Although only few studies can illustrate a link between vitamin D activity and DENV infection or disease, these reports have provided preliminary epidemiological evidence supporting this novel hypothesis. Initially, it was reported that heterozygosity in the VDR gene was correlated with progression of dengue. It was shown in a small Vietnamese population where dengue is endemic that the low frequency of a dimorphic (T/t) “t” allele in the VDR gene was associated with dengue disease severity, suggesting a protective role of VDR activity against dengue disease progression [12]. Variations in VDR have also been associated with susceptibility to osteoporosis in humans and with reduced risk of tuberculosis and persistent hepatitis B virus infections [150-152], highlighting the importance of VDR variations in signaling and immune protection. Accordingly, a study revealed the association of the “T” allele with DHF, by showing that the “T” allele codes for a longer length VDR that is the least active form of VDR. Since vitamin D is known to suppress TNF-a, it is possible that such inappropriate VDR signaling may contribute to higher levels of inflammation, enhancing the susceptibility to severity of the disease [10]. Although the modulatory effect of vitamin D during DENV infection and disease has not been widely tested in human populations, initial studies have associated the effect of oral 25(OH)D3 supplementation with antiviral responses, resistance, and overcoming of the disease. Specifically, a study reported the case of five DF patients that ameliorated the signs and symptoms of the disease, improving the overall clinical conditions and reducing the risk of disease progression [11]. Interestingly, this may be linked to other clinical approaches where oral supplementation with vitamin D enhanced the antiviral response to HCV [63], another RNA virus belonging also to the family Flaviviridae.
The potential antiviral mechanism of vitamin D against DENV has yet not been fully explored; however, certain reports support the proposal that vitamin D could perform anti-DENV effects and immunoregulatory functions on innate immune responses [10-12]. In line with this, the effect of vitamin D treatment of human monocytic cell lines on DENV infection was recently reported [72]. The authors showed that cell exposure to 1,25(OH)2D3 resulted in a significant reduction of DENV-infected cells, a variable modulation of TLR2 and TLR4, and reduced levels of secreted proinflammatory cytokines such as TNF-a, IL-6, and IL-1 after infection [72]. The molecular mechanisms by which vitamin D can elicit an antiviral and anti-inflammatory role towards DENV have not been fully described, and although we observed that monocyte-derived macrophages differentiated in the presence of 1,25(OH)2D3 are less susceptible to DENV infection and express lower levels of mannose receptor restricting binding of DENV to target cells (manuscript in preparation), further studies are required to confirm that vitamin D treatment confers both anti-inflammatory and antiviral responses . Another interesting mechanism that could support the antiviral activity of vitamin D is the VDR-induced regulation of miRs via VDRE. This has been demonstrated for some miRs, such as let-7a (Table 2), whose pri-miR has multiple VDR/RXR binding sites that could potentially be regulated by vitamin D [67, 142]. miR let-7a belongs to a highly conserved family of miRs that contains other miRs previously reported to inhibit DENV replicative activity, such as let-7c [68]. Besides the members of the let-7 family, other miRs have also been associated with suppression of DENV infection and the inflammatory responses against the virus, as discussed below.
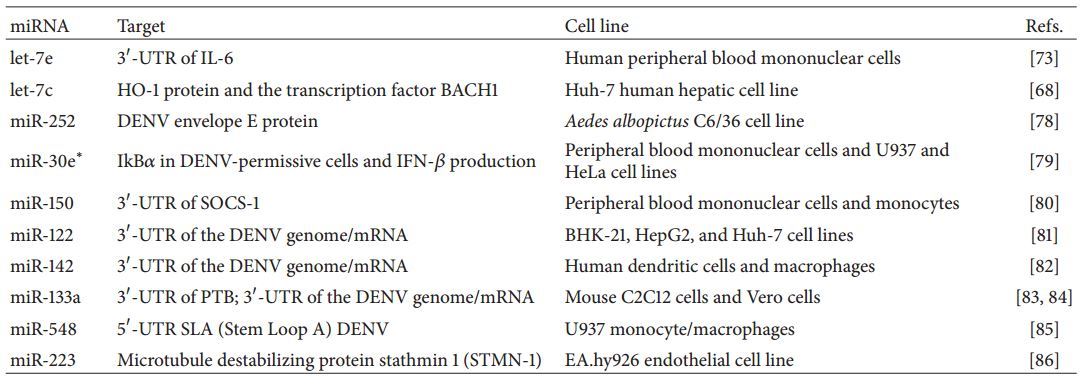
MicroRNAs in DENV Infection. Viruses strictly depend on cellular mechanisms for their replication; therefore, there is an obligatory interaction between the virus and the host RNA silencing machinery. Although virus-derived small interfering RNAs may induce changes in cellular mRNA and miR expression profiles to induce replication, cellular miRs can also target viral sequences or induce antiviral protein expression to inhibit viral replication and translation [153]. Indeed, during DENV infection, several cellular miRs have been reported to have an effect on the replicative activity of the virus and the permissiveness of the host cells. Although some host miRs can also enhance DENV replication [81, 154], here we highlight the miRs affecting DENV replicative activity and modulating the immune response (Table 4).
The expression levels of different miRs regulated during DENV infection have been screened in the hepatic cell line Huh-7. This approach identified miR let-7c as a key regulator of the viral replicative cycle that affects viral replication and the oxidative stress immune response through the protein Heme Oxygenase-1 (HO-1) by activating its transcription factor BACH1 (Basic Leucine Zipper Transcription Factor- 1) [68]. In addition, it was recently reported that, after DENV-2 infection of the C6/36 cell line, endogenous miR- 252 is highly induced and associated with a decreased level of viral RNA copies. This antiviral effect was explained by the fact that miR-252 targets the DENV-2 E protein gene sequence, downregulating its expression and therefore acting as an antiviral regulator [78]. Although DENV can escape the immune system by decreasing the production of type I IFN due to DENV nS5 and NS4B activity [42, 97], DENV infection also induces the upregulation of the cellular miR- 30e* that suppresses DENV replication by increasing IFN– production. This antiviral effect of miR-30e* depends mainly on NF-kB activation by targeting the NF-kB inhibitor ItcBa in DENV-permissive cells [79]. This antiviral effect induced by signaling of type I IFN is also promoted by miR-155 that has been reported to control virus-induced immune responses in models of infection with other members of the Flavivirus genus such as HCV [155-157]. In this latter model, the antiviral effect greatly depended on miR-155 targeting SOCS-1. This observation is in accordance with a study in which elevated expression of miR-150 in patients with DHF was correlated with suppression of SOCS-1 expression in monocytes [80] that in turn could be linked to the fact that vitamin D controls inflammatory responses through modulation of SOCS by downregulating miR-155 [20].
Although it has remained unclear whether endogenous miRs can interfere with viral replicative activity by targeting DENV sequences or viral mRNAs, some experimental approaches have shown the importance of miRs in restricting viral replication through this mechanism [85,158-160]. Some artificial miRs (amiRs) have been described as targeting the highly conserved regions of the DENV-2 genome and promoting efficient inhibition of virus replication [158]. Using DENV subgenomic replicons carrying the specific miR recognition element (MRE) for miR-122 in the 37-UTR of the DENV genome/mRNA, some authors have shown that the liver-specific miR-122 suppresses translation and replication of DENV by targeting this MRE sequence [81]. Likewise, the insertion of the MRE for the hematopoietic specific miR-142 into the DENV-2 genome restricts replication of the virus in DCs and macrophages, highlighting the importance of this hematopoietic miR in dissemination of the virus [82]. In addition, DENV replication is enhanced by the interaction of the viral genome 37-UTR and the host polypyrimidine tract binding (PTB) protein that translocates from the nucleus to the cytoplasm facilitating DENV replication [36, 161, 162]. However, the PTB mRNA 37-UTR contains MREs that can be targeted by miR-133a, providing a mechanism for the downregulation of the PTB protein expression levels [163]. Moreover, in our group, we found that miR-133a contains target sites in the 37-UTR sequence of the 4 DENV serotypes and that overexpression of miR-133a in Vero cells was associated with decreased DENV-2 replication activity [84]. All these data suggest a possible antiviral mechanism via miR-133a targeting the PTB protein mRNA and the DENV 37-UTR sequence. Furthermore, we also showed that miR- 744 and miR-484 can downregulate DENV replication by targeting the 37UTR of the DENV RNA genome [Betancur et al., submitted]. In addition, the cellular miR-548g-3p has been identified as displaying antiviral activity by targeting the 57-UTR SLA (Stem Loop A) promoter of the four DENV serotypes, thus, repressing viral replication and expression of viral proteins, independently of interferon signaling [85]. Moreover, overexpression of miR-223 inhibited replication of DENV in an endothelial cell-like cell line. The authors showed that miR-223 inhibits DENV by negatively regulating the microtubule destabilizing protein stathmin 1 (STMN-1) that is crucial for reorganization of microtubules and later replication of the virus. In addition, this study identified that the transcription factors C/EBP-a and EIF2 are regulators of miR-223 expression after DENV infection [86].
Although little is known regarding the variations in miR expression in DENV-infected individuals, a recent study showed the expression profile of the miRs in blood samples of DEN-infected patients. The authors report 12 miRs that were specifically altered upon acute dengue and 17 miRs that could potentially be associated with specific dengue-related complications [164]. In addition, another profiling study reported abundance changes in the expression of some miRs in DENV- infected peripheral blood monocytes. Importantly, let-7e was among the miRs with the most significant regulation which, besides anti-DENV activity, may be of crucial importance for the modulation of inflammatory responses. Specifically, let-7e shares matching sequences with the 37UTR mRNA of IL-6 and CCL3, as well as of other cytokines, highlighting a key role of miRs in immune response homeostasis during DeNv infection (Figure 1) [67, 73, 86]. Likewise, miR-223 that also shares antiviral activity against DENV has been shown to have an important effect on the inflammatory response by regulating IL- and IL-6 through IKKa and MKP-5 [86, 165, 166], stressing its potential contribution in DENV pathogenesis control. Since a link between vitamin D and miR expression has been established, but no reports discuss their combined implications for DENV antiviral and inflammatory response, we hypothesized here a vitamin D and miR interplay that could modulate DENV pathogenesis, opening new horizons in the therapeutic field of dengue disease.
Concluding Remarks and Future Perspectives
Severe dengue disease symptoms and DENV infection are characterized by overproduction of proinflammatory cytokines driven mainly by activation of several PRRs [29]. Here, we hypothesize that vitamin D may contribute to avoiding DENV infection and disease progression, especially through the modulation of miRs/TLRs that enhance the antiviral activity and regulate the inflammatory response.
Although vitamin D’s antiviral mechanism has not been fully elucidated, it may be linked to vitamin D’s ability to control the permissiveness of DENV target cells and the virus-induced proinflammatory responses [72]. However, a better understanding of these mechanisms is required to provide interesting clues regarding DENV pathogenesis and dengue disease treatment. Certainly, epidemiological and experimental evidence describe an overall positive vitamin D-related immune effect in which increased levels of vitamin D and variants in the VDR receptor are associated with reduction of viral replication, decreased risk of infection, lower disease severity, and better outcome of the dengue symptoms [9-12, 72]. Additionally, the emerging relationships between vitamin D, the TLR signaling pathway, and its regulation by miRs are beginning to gain critical importance in infectious diseases. Indeed, as discussed above, several DENV infection studies have started to illustrate these vitamin D regulatory features that could be key mechanisms for the control of virus replication and homeostasis of the inflammatory response, thus making this hormone a special candidate for therapeutic strategies [127]. Although most of the studies have focused on the effects of vitamin D induced in dendritic cells and macrophages, others have also described the same immunoregulatory effects on other cell populations of the immune system such as CD8+ T cells, NK cells, and B cells [167-169] suggesting their impact not only on DENV target cells but also at the level of cells associated with virus clearance. All the data discussed here suggest that vitamin D could constitute a strong potential strategy to modulate the “cytokine storm” that occurs during ongoing DENV infections and the progression to severe states of the disease. Although it is important to note that such a global effect on the inflammatory activity could weaken the host response to other opportunistic pathogens, it has been suggested that while vitamin D may reduce inflammatory markers during viral infections, it also exerts protective effects against coinfections with other opportunistic pathogens [14,106]. Moreover, its clinical effectiveness has been tested by improving the overall physical condition of DENV patients and reducing the progression of the disease [11]. Although incoming supplementary trials are required to fully elucidate the therapeutic relevance of vitamin D, it is evident that this hormone maybe an excellent alternative of a natural immune-regulatory agent capable of modulating the innate immune response against DENV, which will provide crucial information to understand and design strategies to treat and control progression of dengue disease. Although further experimental studies are required to boost the understanding of vitamin D in the regulation of inflammation and antiviral response against DENV infection, the information discussed above highlights the features of vitamin D in immune regulation as an exciting research field and as an efficient and low-cost therapeutic procedure against DENV and possibly other viral infections.
Competing Interests: The authors declare that they have no competing interests.
Acknowledgments: The authors thank Anne-Lise Haenni for reading the paper and for her constructive and valuable comments. This study was supported by COLCIENCIAS, Grant no. 111556933443, and Universidad de Antioquia, UdeA, CODI (Mediana Cuantla), Acta 624.
