35,000 IU vitamin D weekly during 3rd quarter pregnancy – RCT
Pharmacokinetics of High-Dose Weekly Oral Vitamin D3 Supplementation during the Third Trimester of Pregnancy in Dhaka, Bangladesh
Nutrients 2013, 5(3), 788-810; doi:10.3390/nu5030788
Daniel E. Roth 1,†,[email protected] , Abdullah Al Mahmud 2 , Rubhana Raqib 2 , Evana Akhtar 2 , Robert E. Black 1 and Abdullah H. Baqui 1,2
1 Department of International Health, The Johns Hopkins Bloomberg School of Public Health, 615 North Wolfe Street, Baltimore, MD 21205, USA
2 International Center for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), GPO Box 128, Dhaka 1000, Bangladesh † Present Address: Division of Paediatric Medicine, The Hospital for Sick Children and University of Toronto, 555 University Avenue, Toronto, Ontario, M5G 1X8, Canada.
(This article belongs to the Special Issue Vitamin D and Human Health)
Vitamin D response
NH = not pregnant (much higher response to 35,000) solid line
PH = pregnant, higher dose (35,000 IU weekly)
PL = Pregnant and low (14,000 IU weekly)_ _ _ _ _ _ _ _
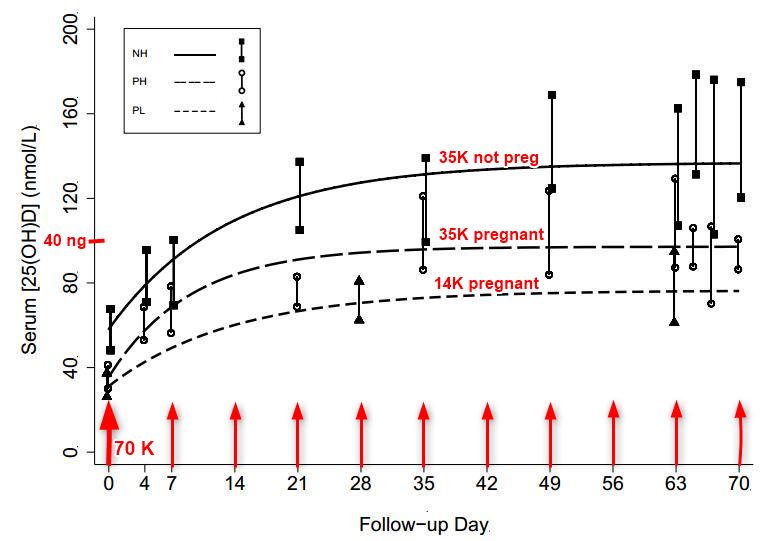
Note: Much faster response than most studies - perhaps because of weekly instead of daily dosing?
A pharmacokinetic study was conducted to assess the biochemical dose-response and tolerability of high-dose prenatal vitamin D3 supplementation in Dhaka, Bangladesh (23°N). Pregnant women at 27–30 weeks gestation (n = 28) were randomized to
70,000 IU once + 35,000 IU/week vitamin D3 (group PH: pregnant, higher dose ) or
14,000 IU/week vitamin D3 ( PL: pregnant, lower dose ) until delivery.
A group of non-pregnant women (n = 16) was similarly administered 70,000 IU once + 35,000 IU/week for 10 weeks ( NH: non-pregnant, higher-dose ).
Rise (∆) in serum 25-hydroxyvitamin D concentration ([25(OH)D]) above baseline was the primary pharmacokinetic outcome.
Baseline mean [25(OH)D] were similar in PH and PL (35 nmol/L vs. 31 nmol/L, p = 0.34).
A dose-response effect was observed: ∆[25(OH)D] at modeled steady-state was 19 nmol/L (95% CI, 1 to 37) higher in PH vs. PL (p = 0.044). ∆[25(OH)D] at modeled steady-state was lower in PH versus NH but the difference was not significant (−15 nmol/L, 95% CI −34 to 5; p = 0.13). In PH, 100% attained [25(OH)D] ≥ 50 nmol/L and 90% attained [25(OH)D] ≥ 80 nmol/L; in PL, 89% attained [25(OH)D] ≥ 50 nmol/L but 56% attained [25(OH)D] ≥ 80 nmol/L. Cord [25(OH)D] (n = 23) was slightly higher in PH versus PL (117 nmol/L vs. 98 nmol/L; p = 0.07).
Vitamin D3 was well tolerated; there were no supplement-related serious adverse clinical events or hypercalcemia.
In summary, a regimen of an initial dose of 70,000 IU and 35,000 IU/week vitamin D3 in the third trimester of pregnancy was non-hypercalcemic and attained [25(OH)D] ≥ 80 nmol/L in virtually all mothers and newborns. Further research is required to establish the safety of high-dose vitamin D3 in pregnancy and to determine if supplement-induced [25(OH)D] elevations lead to maternal-infant health benefits.
📄 Download the PDF from Vitamin D Life
4. Discussion
This study demonstrated the biochemical dose response to third-trimester high-dose weekly antenatal vitamin D3 supplementation. Among Bangladeshi women with a mean [25(OH)D] of 33 nmol/L, 70,000 IU followed by 35,000 IU/week of vitamin D3 until delivery yielded an average [25(OH)D] that was about 20 nmol/L higher than an antenatal dose of 14,000 IU/week (the IOM vitamin D upper limit at the time the study was conducted). Similar to our conclusions from analyses of single-dose vitamin D3 pharmacokinetics in the same study setting (and involving an overlapping group of participants) [13], we found that the minor differences between pregnant vs. non-pregnant participants receiving the same dose were within the margins of error given the small sample size. However, based on the present analysis, we could not exclude the possibility of a slightly diminished 25(OH)D response to a weekly dose of vitamin D during the third trimester of pregnancy.
To our knowledge, the 35,000 IU/week regimen used in this study is the highest vitamin D3 maintenance dose studied in pregnancy under controlled conditions. Devlin et al. (1986) reported that a daily dose of 1000 IU vitamin D3 administered to 15 French women during the third trimester modestly raised mean maternal serum [25(OH)D] from 55 nmol/L to 65 nmol/L [20]. The largest published study of vitamin D3 supplementation in pregnancy was conducted by Bruce Hollis and colleagues in South Carolina, in which 502 pregnant women at 12 to 16 weeks gestation were randomized to 400 IU/day, 2000 IU/day, or 4000 IU/day vitamin D3 [21]. This population was more vitamin D-replete at baseline (mean [25(OH)D] = 60 nmol/L) compared to the present study. Based on data from the 350 participants (70%) followed until delivery, the 2000 IU/day and 4000 IU/day regimens raised [25(OH)D] to means of 105 nmol/L (rise of 47 nmol/L) and 119 nmol/L (rise of 60 nmol/L), respectively, at one month before delivery [21]. The A[25(OH)D] in the 2000 IU/day group in the Hollis study was similar to the response we observed in the 14,000 IU/day group (equivalent regimen) in the present study, substantiating the consistency of vitamin D3 dose-response modeling across diverse populations of pregnant women. In a separate trial in South Carolina, Wagner et al. reported comparatively less robust responses to 2000 IU/day and 4000 IU/day during pregnancy, which may have been attributable to non-adherence to the supplementation regimen [22].
The lower dose produced a more efficient 25(OH)D response per mcg of vitamin D3 when compared to the high-dose regimen: 0.73 vs. 0.46 nmol/L/mcg/day in the empiric estimates, and 0.90 versus 0.49 nmol/L/mcg/day based on the pharmacokinetic model. These estimates, as well as those from the non-pregnant cohort that received the higher-dose regimen (0.61 nmol/L/mcg/day based on 10th-week data, and 0.63 nmol/L/mcg/day based on the parametric model), were similar to the values conventionally cited for non-pregnant adults: ~0.70 nmol/L/mcg/day [23,24]. However, analyses by Barger-Lux et al. (1998) [25] and Aloia et al. (2008) [24], as well the recent IOM report (2010) [1], have demonstrated that the A[25(OH)D] per mcg is a curvilinear inverse function of vitamin D intake at doses <50 mcg/day, but nearly proportional to intake at >50 mcg/day [24], which may explain the greater observed efficiency of the lower dose.
A unique aspect of this study was the measurement of biochemical parameters between weekly doses at the end of the supplementation period. These data showed an absence of inter-dose perturbations in calcium homeostasis that might have otherwise been missed by sampling serum only at the time of the "trough" [25(OH)D] (i.e., immediately preceding administration of a weekly dose). Although the study may have been too small to detect minor inter-dose fluctuations in [25(OH)D], the data supported the appropriateness of administering weekly doses of 35,000 IU instead of daily administration of 5000 IU.
In pregnant participants, the higher-dose vitamin D regimen had a significant suppressive effect on maternal PTH secretion, relative to the lower dose, as indicated by the change in average PTH concentrations from baseline to delivery, similar to previous observations by Wagner et al. in South Carolina [22]. However, since the role of PTH as a vitamin D status biomarker during pregnancy is unclear [26], the clinical significance of the apparent dose-response effect of vitamin D on PTH requires further study.
Both the higher and lower vitamin D3 regimens administered to pregnant women attained fetal [25(OH)D] > 50 nmol/L. Therefore, in this small sample, we did not observe a clear benefit of the higher-dose over the lower-dose regimen with respect to neonatal vitamin D status . In a related study at the same study site, we observed a mean cord [25(OH)D] of 50 nmol/L (range of 29 to 80 nmol/L) in a group of neonates born to women who had received a single vitamin D3 dose of 70,000 IU at 30 weeks gestation [13], and previous studies in South Asia have found cord serum [25(OH)D] ranging from 17 to 59 nmol/L [27-30].
Appreciable increases in serum calcium in the higher-dose relative to the lower-dose group highlighted a dose-dependent effect of vitamin D3 supplementation on calcium homeostasis. We previously reported that mean serum calcium concentrations rose slightly but significantly during the first week after administration of a single 70,000 IU dose of vitamin D3 in both pregnant and non-pregnant participant groups [13]. However, in the present analyses of weekly-dose vitamin D3, a significant increase in serum [Ca] from baseline was only observed in pregnant women who received the higher dose. Pregnancy is associated with an elevation in the maternal serum concentration of the active vitamin D metabolite, 1,25-dihydroxyvitamin D (1,25(OH)2D) [31,32], which appears to be primarily attributable to classic renal 1a-hydroxylation of 25(OH)D [33]. However, placental trophoblasts and decidual cells [34] are capable of extra-renal 1a-hydroxylation which could theoretically predispose the pregnant woman to exaggerated physiological responses to increases in [25(OH)D] [9]. Similar to the participants who received only a single dose of 70,000 IU [13], maternal serum calcium values in the weekly-dose participants were all below the threshold for defining hypercalcemia used by the IOM in setting the 1997 dietary reference intakes (DRIs) for vitamin D (2.75 mmol/L) [35] and in the revised DRIs in 2010 (2.63 mmol/L) [1]. Cord blood calcium concentrations were also within reference limits, and [25(OH)D] were well below the range that has been associated with toxicity in adults [36] and older children [37]. Pregnancy and newborn clinical outcomes were within the expected range for the study population, but we were unable to draw conclusions from this study regarding clinical effects of vitamin D. Nonetheless, this study together with the recent findings of Hollis and Wagner and colleagues in South Carolina [21,22] demonstrate that vitamin D3 doses during pregnancy up to 25% above the current IOM UL of 4000 IU/day do not induce hypercalcemia, and have not led to any observed short-term clinical adverse effects.
There were several important limitations of this study. First, precision of estimates of pharmacokinetic parameters and between-group comparisons, as well as the generalizability of inferences regarding maternal-fetal safety of high-dose vitamin D supplementation, were limited by the small number of participants, stringent inclusion/exclusion criteria, and enrolment of pregnant and non-pregnant participants at one clinic site. Moreover, the lower-dose pregnancy group had less frequent blood sampling (a cost-savings measure given the relative lack of safety concerns for this group) and only 9 of 14 enrolled women contributed endpoint samples during the 10th week of supplementation. The supplementation period may not have been long enough to ensure that all participants reached a steady-state [25(OH)D]. Conclusions based on comparisons between pregnant and non-pregnant women were tempered by the differences in baseline characteristics, including season of enrolment and the relatively higher socioeconomic status of the non-pregnant participants. In addition, there were too few participants to consider modifiers of A[25(OH)D]. Most importantly, the present results do not yet provide sufficient evidence that the regimens studied are beneficial or safe for use in clinical or public health practice; rather, they serve to inform application of these dose regimens in future research studies.
5. Conclusions
This detailed analysis of the response to high-dose weekly vitamin D3 administered during the third-trimester of pregnancy demonstrated a dose-responsiveness to oral vitamin D3 in Bangladeshi women that echoed observations in other settings, and was generally in accordance with established pharmacokinetic characteristics of vitamin D3. Nonetheless, increases in the mean calcium concentration (within the normal range) and suppression of PTH secretion among pregnant women receiving the higher-dose regimen (70,000 IU initial dose followed by weekly doses of 35,000 IU) highlighted the physiological impact of the intervention and the need to cautiously address potential pregnancy-specific sensitivities to vitamin D supplementation.
Prior to undertaking large trials to test the effects of prenatal micronutrient interventions on pregnancy and birth outcomes, preliminary dose-finding and safety studies are essential, particularly when the intervention is a fat-soluble vitamin at a dose above the conventional upper limit of tolerability (i.e., 4000 IU/day for vitamin D, as established by the Institute of Medicine [1]). The most direct application of the present observations is to guide the design of future trials of vitamin D3 (at doses up to 35,000 IU per week) aimed at confirming safety and establishing the health benefits of antenatal vitamin D supplementation in South Asia, where many potentially vitamin D-responsive outcomes (e.g., infant growth and infectious disease morbidity) are major public health priorities. Following from our preliminary pharmacokinetic studies, we have conducted a placebo-controlled trial of 35,000 IU/ week during the third trimester (n = 160), with follow-up of infants to monitor growth to one year of age (NCT01126528). Future trials in Dhaka will address the dose-dependency of the effects of prenatal vitamin D supplementation on infant growth and morbidity.
See also Vitamin D Life
Overview Pregnancy and vitamin D has the following summary
{include}
Pregnancy category starts with
{include}
Healthy pregnancies need lots of vitamin D has the following summary
{include}
The articles in Pregnancy AND Intervention are here:
{category}
The articles in Pregnancy AND Meta-analysis are here:
{category}
